OGT, a conserved O-GlcNAc transferase, modifies the function, stability, and localization of intracellular proteins by O-GlcNAcylation of their serine and threonine residues. OGT and OGA work together to maintain cellular O-GlcNAc homeostasis, which is crucial for a range of cellular functions such as metabolism, stress responses, and proteostasis. The mammalian cell cycle regulator host cell factor C1 is proteolytically cleaved and activated by OGT (HCF-1). In C. elegans GABA neurons, it also has non-catalytic activities in epithelial adherence junctions and an EEl-1-dependent E3 ubiquitin ligase complex. In most metazoans, knocking down OGT is lethal at either the single-cell or developmental level.
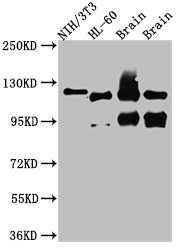
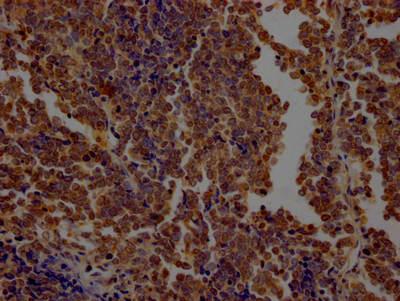
The recombinant OGT antibody is a monoclonal antibody molecule expressed by using recombinant DNA and protein engineering technology to clone the genes encoding the OGT antibody into a plasma vector and then by transfecting the vector clone into the appropriate recipient mammalian cells for production. It was purified using Affinity-chromatography. And it shows reactivity with OGT protein from Human, Mouse, Rat. This recombinant OGT antibody can be used in the ELISA, WB, IHC.