The Wnt signaling pathways, highly conserved amongst various species, are a group of signal transduction pathways made of proteins that pass signals into a cell through cell surface receptors, and are essential in developmental processes. Accumulating studies have demonstrated that abnormal activated Wnt singling pathway is closely related to the development of cardiovascular disease, liver fibrosis and cancer; while the down-regulation of key molecules in the Wnt signaling pathway leads to another type of disease, such as familial exudative vitreoretinopathy, Alzheimer's disease and osteoporosis. Therefore, further research on the Wnt signaling pathway, discovery and identification of new molecules affecting the Wnt signaling pathway is very important for understanding the mechanism of early development of animals and treatment-related diseases.
1. The history of the Wnt signaling pathway
The Wnt gene was originally identified as a site for preferential integration of mouse mammary tumor virus by Nusse and Varmus, which discovered in 1982. This gene, named the Int gene at that time, is an oncogene and transmits the information of proliferation and differentiation between cells. It was subsequently found an orthologous gene (known as wingless gene) in Drosophila. The name of Wnt is derived from Int and wingless gene. With the further research, it has been found that the Wnt gene family is very large, and most of them are multi-gene family. Its gene structure is highly conserved from low invertebrates to vertebrates, and Its homologous sequence is about 27-83%. The Wnt protein, encoded by the Wnt gene, initiates an intracellular signaling pathway, conducts growth stimulation signals, and participates in various developmental mechanisms, such as cell differentiation, migration, and proliferation of cell fate. Because its promoter protein is WNT protein, it is named Wnt signaling pathway. In a word, Wnt gene plays a key role in animal growth and development[1].
2. Wnt family
Wnt includes a diverse family of secreted lipid-modified signaling glycoproteins that are 350-400 amino acids in length[2]. The type of lipid modification that occurs on these proteins is palmitoylation of cysteines in a conserved pattern of 23-24 cysteine residues[3]. Palmitoylation is a necessary in Wnt signaling pathway, because it initiates targeting of the Wnt protein to the plasma membrane for secretion and it allows the Wnt protein to bind its receptor due to the covalent attachment of fatty acids. Wnt proteins also undergo glycosylation, which attaches a carbohydrate in order to ensure proper secretion[4]. In Wnt signaling, these proteins activate the different Wnt pathways via paracrine and autocrine routes as ligands.
These proteins are highly conserved across species. They can be found in mice, humans, Xenopus, zebrafish, Drosophila and many others. If you want to know more targets about the Wnt signaling pathway, please click this link.https://www.cusabio.com/pathway/Wnt-signaling-pathway.html.
Table 1. the members of Wnt family in different species
|
Species
|
Wnt proteins
|
|
Homo sapiens
|
WNT1, WNT2, WNT2B, WNT3, WNT3A, WNT4, WNT5A, WNT5B, WNT6, WNT7A, WNT7B, WNT8A, WNT8B, WNT9A, WNT9B, WNT10A, WNT10B, WNT11, WNT16
|
|
Mus musculus (Identical proteins as in H. sapiens)
|
Wnt1, Wnt2, Wnt2B, Wnt3, Wnt3A, Wnt4, Wnt5A, Wnt5B, Wnt6, Wnt7A, Wnt7B, Wnt8A, Wnt8B, Wnt9A, Wnt9B, Wnt10A, Wnt10B, Wnt11, Wnt16
|
|
Xenopus
|
Wnt1, Wnt2, Wnt2B, Wnt3, Wnt3A, Wnt4, Wnt5A, Wnt5B, Wnt6, Wnt7A, Wnt7B, Wnt8A, Wnt8B, Wnt10A, Wnt10B, Wnt11, Wnt11R
|
|
Danio rerio
|
Wg, DWnt2, DWnt3/5, DWnt4, DWnt6, WntD/DWnt8, DWnt10
|
|
Hydra
|
hywnt1, hywnt5a, hywnt8, hywnt7, hywnt9/10a, hywnt9/10b, hywnt9/10c, hywnt11, hywnt16
|
|
C. elegans
|
mom-2, lin-44, egl-20, cwn-1, cwn-2
|
*This data of table 1 is derived from Wikpedia
3. Three types of the Wnt signaling pathways
The Wnt signaling pathway is a complex regulatory network that have been characterized with three branches, including the canonical Wnt pathway, the planar cell polarity(PCP) pathway, and the Wnt/Ca2+ pathway. All three pathways are activated though binding a Wnt-protein ligand to a Frizzled family receptor, which transmits the biological signal to the Dishevelled protein inside the cell. The canonical Wnt pathway leads to regulation of gene transcription, and is thought to be negatively regulated in part by the SPATS1 gene. The noncanonical planar cell polarity pathway regulates the cytoskeleton that is responsible for the shape of the cell. The noncanonical Wnt/calcium pathway regulates calcium inside the cell.
Canonical Wnt pathway
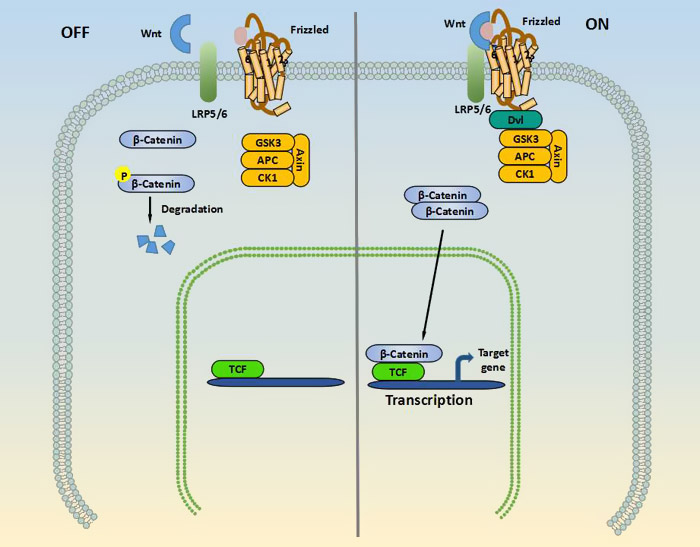
Canonical Wnt pathway, also known as WNT/β-Catenin Signaling pathway, activates transcription of target genes through stabilization of β-catenin in the nucleus. β-Catenin is a protein of 80 kDa that is a vital molecule in the WNT/β-Catenin Signaling pathway[5]. The function of this pathway during embryonic development has been originally elucidated by experimental analysis of axis development in the frog Xenopus laevis and of segment polarity and wing development in the fly Drosophila melanogaster. As shown in the picture 1, In the "Off" state, β-Catenin is bound in a β-Catenin destruction complex which is consist of glycogen synthase kinase 3β (GSK3β), axin, adenomatous polyposis coli (APC) and casein kinase-1 (CK-1). β-catenin is phosphorylated by the kinases in this complex, thereby targeting this complex for degradation by the ubiquitin proteasome system. In the "On" state, the receptor complex containing frizzled and LRP5/6 binds to WNT, which recruits the disheveled (DVL) protein to the plasma membrane. Meanwhile, several components of the β-catenin destruction complex are recruited to the membrane, which prevents the phosphorylation of β-catenin. Therefore, this protein can now accumulate in the cytoplasm and translocate to the nucleus to associate with transcription factors and stimulate the transcription of WNT target genes such as cycline-D1, c-myc, and axin2.
Planner cell polarity pathway
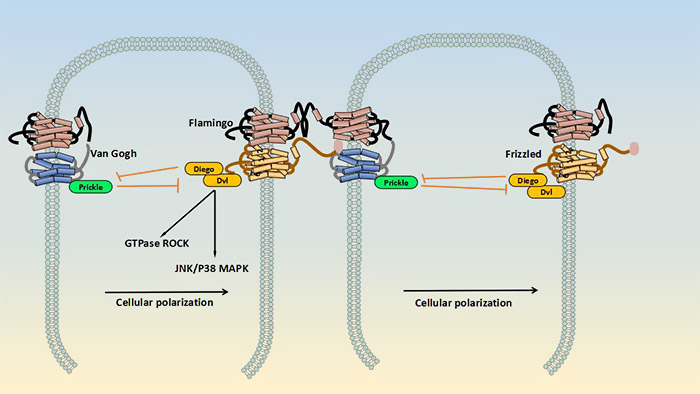
The planner cell polarity pathway, also known as non-canonical Wnt signaling pathway, does not involve β-catenin. It does not use LRP-5/6 as its co-receptor and regulates cytoskeletal rearrangement by activation of JNK (c-Jun N-terminal kinase). As shown in the picture2, in this pathway, there are two receptor complexes which formed at the opposite sides of a cell. On one side, a complex consists of frizzled, Flamingo/Celsr, disheveled (Dvl), and Diego/Diversin, whereas the other side the complex contains Van Gogh/Vang, Flamingo/Celsr and Prickle. The extracellular parts of these complexes interact, thereby controlling the cellular polarization via activation of JNK/p38 MAPK, small GTPase and Rho-associated kinase (ROCK) signaling. Although the role of Wnt in this signal transduction cascade is only partially understood, this protein may interfere with the Vangl1-FZD interaction. This could subsequently disturb the balance between the signaling of the Vang1/Prickle/Celsr complex and the FZD/DVL/Diego/Celsr complex[6][7].
Wnt/Ca2+ pathway
The Wnt/Ca2+ pathway, activated by Wnt5a and Wnt11, affects cell adhesion and related gene expression by releasing intracellular Ca2+. The Wnt/Ca2+ pathway interacts with a typical Wnt/β-cantenin signaling pathway. Ca2+ is one of the most abundantly used second messengers. Currently, emerging evidence demonstrated that WNT/Ca2+ signaling pathway can employ two pathway, one involving phospholipaseC-mediated production of inositol triphosphate and diacylglycerol and the other via the cyclic GMP selective phosphodiesterase and p38-MAPK[8]. The Wnt/Ca2+ signaling pathway is involved in regulating cytoskeletal rearrangements, cellular adhesion, and other developmental processes, such as dorsoventral patterning and tissue separation in embryos[9].
4. Wnt signaling pathway and disease
Wnt signaling pathway plays divergent roles during development, normal homeostasis and disease. In this part, we have compiled some diseases related to Wnt signaling pathway in recent years.
Wnt signaling pathway and Vascular Disease
Atherosclerosis is a prime cause of cardiovascular diseases. It is a chronic inflammatory condition that can lead to an acute clinical event due to plaque rupture and thrombosis. In 2007, Mani et al. offered the first evidence that linked WNT signaling to atherosclerosis by identifying a mutation in the LRP6 gene in a family with autosomal dominant early coronary disease, hypertension, hyperlipidemia, and osteoporosis. The functional significance of the corresponding mutation - an amino acid substitution (Cys-Arg) that affects an epidermal growth factorlike domain - was further confirmed in vitro in cells transfected with the mutant LRP6 and WNT reporter[10]. Endothelial dysfunction is a well-established hallmark of atherosclerosis development. Accumulating studies have suggested that ECs from diabetic patients had higher WNT5A expression than from nondiabetic patients and this was associated with higher levels of activated c-jun N-terminal kinase (JNK)[11]. Wnt signaling is not only an important key pathway for atherosclerosis development but also seems to be of importance for its regression. The activation of Wnt signaling in atherosclerosis is likely to contribute to the inflammatory response, endothelial dysfunction, and VSMC proliferation and appears to involve both β-catenin-mediated and non-β-catenin-mediated WNT signaling.
Wnt signaling pathway and Cardiac Disease
Myocardial infarction (MI) is one of the most prevalent acute cardiovascular events. It usually occurs as a consequence of the progression of an atherosclerotic plaque toward an unstable phenotype, where the fibrous cap ruptures and releases the content of the plaque into the circulation. Sizeable researches have reported that the expression of multiple genes of the WNT pathway are suppressed in a mouse model of MI which the underlying pathology of human infarction is mimicked by a surgical ligation of a coronary artery, including the upregulation of WNT2, -4, 10-b, and -11 expression and the downregulation of WNT7B[12][13][14][15]. Emerging evidence showed that the effect of modulation of WNT expression or secretion on infarct healing was addressed. In a mouse model of cardiomyocyte-specific overexpression of WNT10B, Applying coronary artery ligation and cryoinjury increases neovascularization of the infarct zone, decreases the size of scar with fewer myofibroblasts and improves ventricular function[16].
Wnt signaling pathway and Cancer
Cancer is a collective term for a series of diseases with very high mortality rates, including breast cancer, colon cancer, liver cancer, Ovarian Cancer, et al. Accumulating evidence indicated that aberrant regulation of the Wnt signaling pathway is a prevalent phenomenon in cancer biology[17]. Wnt/beta-catenin signaling plays an essential role in colon cancer. Song S, et al. has reported that Galectin-3 (a beta-galactoside-binding protein) mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity[18]. Furthermore, macrophage-derived IL-1beta, which can induce phosphorylation of GSK3beta, stabilize beta-catenin, enhance T-cell factor (TCF)-dependent gene activation and induce the expression of Wnt target genes in tumor cells, stimulates Wnt signaling and growth of colon cancer cells[19]. In recent years, for the treatment of cancer, Wnt signaling pathway has been a effective target. As the Riley RS's study shown, Frizzled7 antibody-functionalized nanoshells enable multivalent binding for Wnt signaling inhibition in triple negative breast cancer cells[20]. and Niclosamide-conjugated polypeptide nanoparticles inhibit Wnt signaling and colon cancer growth[21].
5. The latest research of Wnt Signaling Pathway
In this part, we are listing some latest researches about Wnt Signaling Pathway.
#1 Luo L, Hong X, et al. reported that sulfur dioxide attenuates hypoxia-induced pulmonary arteriolar remodeling via Dkk1/Wnt signaling pathway. Please click here to view the article.
#2 Chattopadhyay S, Chaklader M, et al. demonstrated that aberrant Wnt signaling pathway in the hematopoietic stem/progenitor compartment in experimental leukemic animal. Please click here to view the article.
#3 Li S, Han Z, et al. showed that Inhibition of DNMT suppresses the stemness of colorectal cancer cells through down-regulating Wnt signaling pathway. Please click here to view the article.
References
[1] Tsukamoto AS, Grosschedl R, et al. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice[J]. Cell. 1988, 55:619–625.
[2] Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development[J]. Genes & Development. 1997, 11 (24): 3286–305.
[3] Logan CY, Nusse R. The Wnt signaling pathway in development and disease[J]. Annual Review of Cell and Developmental Biology. 2004, 20: 781–810.
[4] Kurayoshi M, Yamamoto H,et al. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling[J]. The Biochemical Journal. 2007, 402 (3): 515–23.
[5] Sébastien Foulquier, Evangelos P. Daskalopoulos, et al. WNT Signaling in Cardiac and Vascular Disease[J]. Annu Rev Cell Dev Biol. 2004, 20:781-810.
[6] Yang Y and Mlodzik M. Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt)[J]. Annu Rev Cell Dev Biol. 2015, 31:623–646.
[7] Wu J, Roman AC, et al. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila[J]. Nat Cell Biol. 2013, 15:1045–1055.
[8] Ma L and Wang HY. Mitogen-activated protein kinase p38 regulates the Wnt/cyclic GMP/Ca2+ non-canonical pathway[J]. J Biol Chem. 2007, 282:28980–28990.
[9] A.D. Kohn, R.T. Moon, Wnt and calcium signaling: beta-catenin-independent pathways[J]. Cell Calcium. 2005, 38:439–446.
[10] Mani A, Radhakrishnan J, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors[J]. Science. 2007, 315:1278–1282.
[11] Bretón-Romero R, Feng B, et al. Endothelial Dysfunction in Human Diabetes Is Mediated by Wnt5a-JNK Signaling[J]. Arterioscler Thromb Vasc Biol. 2016, 36:561–569.
[12] Barandon L, Couffinhal T, et al. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA[J]. Circulation. 2003, 108:2282–2289.
[13] Aisagbonhi O, Rai M, et al. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition[J]. Dis Model Mech. 2011, 4:469–483.
[14] Paik DT, Rai M, et al. Wnt10b gain-of-function improves cardiac repair by arteriole formation and attenuation of fibrosis[J]. Circ Res. 2015, 117:804–816.
[15] Morishita Y, Kobayashi K, et al. Wnt11 gene therapy with adeno-associated virus 9 improves recovery from myocardial infarction by modulating the inflammatory response[J]. Sci Rep. 2016, 6:21705.
[16] Paik DT, Rai M, et al. Wnt10b gain-of-function improves cardiac repair by arteriole formation and attenuation of fibrosis[J]. Circ Res. 2015, 117:804–816.
[17] Song S,Mazurek N, et al. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity[J]. Cancer research. 2009, 69(4):1343-9.
[18] Kaler P,Augenlicht L, et al. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3[J]. Oncogene. 2009, 28(44):3892-902.
[19] Riley RS,Day ES. Frizzled7 Antibody-Functionalized Nanoshells Enable Multivalent Binding for Wnt Signaling Inhibition in Triple Negative Breast Cancer Cells[J]. Small. 2017,13,26.
[20] Bhattacharyya J,Ren XR, et al. Niclosamide-conjugated polypeptide nanoparticles inhibit Wnt signaling and colon cancer growth[J]. Oncogene,2017.






Comments
Leave a Comment