On August 23, 2022, Sinocelltech Group Ltd. from China's NMPA has received marketing approval for its recombinant chimeric anti-CD20 monoclonal antibody--Ripertamab (SCT-400), to treat patients with CD20-positive diffuse large B-cell lymphoma. Ripertamab is also first NMPA approval for a homegrown anti-CD20 MAb. The constant region of Ripertamab is G1m (1,17), the most common natural IgG1 allotype extracted from blood B cells of Chinese healthy volunteers. Ripertamab differs from Rituximab by only one amino acid (V219A in the CH1 domain of the heavy chain), which brings a superior safety profile [1]. Successive studies have confirmed that anti-CD20 therapy is also associated with the course of COVID-19, which is inducing long-term growth for anti-CD20 monoclonal market.
Each designed CD20 drugs with modifications to structure aimed at further improving efficacy. In parallel, many novel CD20 drugs have entered the clinical research, including bispecific antibody, biosimilars, ADC, CAR-T, small molecule chemistries and other innovative drugs. Especially for anti-CD20 monoclonal antibodies, there are more than 100 clinical trials. Top manufacturers and key players in anti-CD20 monoclonal antibodies market include Genmab, Amgen, Roche, Pfizer etc. Apparently, anti-CD20 monoclonal antibodies market is rapidly increasing and it is poised to grow by $9.05 bn during 2022-2026!
CD20 is a non-glycosylated phosphoprotein located on B cells and is mainly expressed at the pre-B cell to mature B cell stage. The CD20 molecule is a hydrophobic, 4-transmembrane protein consisting of 297 amino acid residues with a relative molecular mass of approximately 33 x 103. The expression of CD20 is highly variable between different B cell malignancies [2-3]. The molecular function of CD20 is related to the signaling propensity of the B cell receptor (BCR). CD20 has been shown to be associated with a variety of other surface proteins on B cells (e.g. CD40, CD53, CD81 and CD82). It has been shown that some epigenetic factors (EZH2, HDAC1/2, HDAC6, Sin3A-HDAC1 complex) and transcription factors (USF, OCT1/2, PU.1, ELK1, PiP, ETS1, and SP1, NFκB, FOXO1, and SMAD2/3) can also regulate CD20 expression [4-6].
It is widely recognized that CD20 is a surface-specific molecular marker of human B-lymphocytes, which mediates in the proliferation and differentiation of B-lymphocytes. CD20 is expressed in a vast majority of mature B-cell neoplasms. The CD20 protein has no known natural ligand to interfere with its binding to antibodies, and there is no significant internalization and shedding of CD20 after binding to antibodies or antigenic modulation due to binding to antibodies, making it an ideal target for the treatment of B-cell lymphoma. Currently, anti-CD20 monoclonal antibody drug is an important strategy for the treatment of B-cell non-Hodgkin's lymphoma (Figure 1) [4]!

Figure 1. Anti-CD20 monoclonal antibody drugs for BCL treatment [4]
CD20 exists as an oligomer on the surface of B lymphocytes. It has been demonstrated that CD20 forms tetramers on the surface of B cells. B cell antigen receptor (BCR) signaling initiates sustained cellular calcium influx necessary for the development, differentiation, and activation of B lymphocytes. The interaction of CD20 with the membrane type IgM (sIgM) of the B cell antigen receptor (BCR) has been identified. Furthermore, after activation, BCR-CD20 complexes dissociated, phosphoproteins and calmodulin-binding proteins were transiently recruited to CD20, which provide new evidence of the involvement of CD20 in signaling downstream of the BCR [2-4].
Calcium ions (Ca2+) movement contributes to signal transduction by affecting local electrostatic fields and interacting with proteins to alter their conformations. In many cases, the mobilization of Ca2+ is controlled by intracellular store activation and calcium influx, which is characterized by sustained calcium entry into the cell via plasma membrane-associated “store-operated” calcium entry (SOCE) channels. Calcium-release-activated calcium (CRAC) channel is a primary player in SOCE to increase intracellular calcium concentration in lymphocytes. Accumulating evidence indicated that CD20 is involved in SOCE, responsible for intracellular calcium ion concentration. The intensity and duration of calcium ion movement induced by SOCE will affect the function of the cell after receptor-antigen binding [6-8].
How do anti-CD20 drugs work? Currently, CD20 widely used in cancer treatment is CD20-targeting mAbs. How do anti-CD20 monoclonal antibodies work? A large body of evidence suggests that anti-CD20 monoclonal antibodies can induce tumor killing via various mechanisms are mainly associated with three regulation mechanisms, namely antibody-dependent cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and directly induction of apoptosis by antibody binding to CD20 molecules.
ADCC is also known as antibody-dependent cellular cytotoxicity. ADCC is an immune response where Fc receptor-bearing effector cells recognize and kill antibody-coated target cells that express tumor or pathogen-specific antigens on their surface. CDC is another molecular mechanism for anti-CD20 antibodies, which is an immunological process in which pathogens are killed by damaging their plasma membranes without the involvement of the immune system cells or antibodies. CD20 is an effective immunotherapy target for CD20+ B-cell malignant cells. Besides, CD20 is commonly used in the investigation of lymphomas, leukemias, or other immune and inflammatory diseases, making the CD20 antigen a attractive target for the treatment of diseases such as lymphoma, leukemia, and certain autoimmune diseases (Figure 2) [3, 9-12].
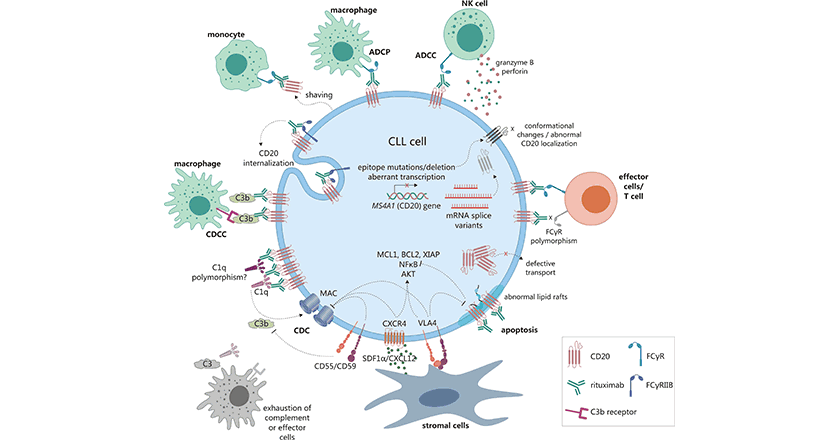
Figure 2. The mechanisms of anti-CD20 monoclonal antibodies in tumors [3]
There are three main forms for the CD20 medication: anti-CD20 monoclonal antibodies, CAR-T and bispecific antibodies. Numerous pharmaceutical companies focus on CD20 monoclonal antibodies. According to the degree of humanization and Fc fragment modification, anti-CD20 monoclonal antibodies can be divided into three generations, of which the first generation is represented by rituximab as a chimeric or mouse-derived monoclonal antibody, the second generation is represented by ovalbumin monoclonal antibody as a humanized monoclonal antibody, and the third generation of anti-CD20 monoclonal antibodies is represented by ovalbumin monoclonal antibody whose Fc fragment has been modified by glycosylation [13-15].
CD20 has been a superb biomarker for immunotherapies targeting B-cell derived diseases. Specifically, Rituximab (RTX), a murine-human chimeric anti-CD20 mAb, was the first anti-CD20 mAb developed as a therapeutic agent. As a monoclonal antibody against CD20, Rituximab has been commonly used for lymphoma treatment. At present, Rituximab in combination with cyclophosphamide/doxorubicin/vincristine/prednisone (R-CHOP) remains the standard frontline strategy for diffuse large B-cell lymphoma. The second-generation anti-CD20 mAbs include ofatumumab, veltuzumab, and ocrelizumab, which are humanized to reduce immunogenicity. The third-generation humanized CD20 mAbs have an engineered Fc region to increase the binding affinity for the FcγRIIIa receptor on immune effector cells. Surely, the development of anti-CD20 monoclonal antibodies is ongoing. Novel agents with different regulation mechanisms are being explored like biospecific antibody, trispecific antibody, ADC, CAR-T, or others. These novel anti-CD20 mAbs may provide further improvement on immunotherapy [13-15].
According to the data from Synapse, 184 novel therapeutic drugs targeting CD20 in clinical trials worldwide (Table 1), over 80% CD20 monoclonal antibodies drugs. As of now, 26 anti-CD20 monoclonal antibodies drugs have been approved by the FDA. In addition, the global manufacturers of anti-CD20 monoclonal antibodies drugs include Amgen, Roche, Novartis AG, Pfizer, Biogen, Genentech and Immunomedics, AbbVie etc. More importantly, the recent years have seen many Chinese pharmaceutical companies stepping up efforts in anti-CD20 monoclonal antibodies like Sinocelltech Group Ltd., Jiangsu Hengrui Pharmaceuticals, InnoCare Pharma, Chinese People's Liberation Army General Hospital, Zai Lab Ltd., BioRay Biopharmaceutical Co., Ltd, and others. The targeted therapies against CD20 are not limited to antibodies, more innovative drugs are being designed such as CD20 dual antibodies, ADC, CAR-T! Obviously, the competition for CD20 monoclonal antibodies drugs looms among global companies, which are estimated to gain market growth in the upcoming years!
| Drugs | Target | Mechanism | Drug Type | Indications | Institutes | R&D status |
|---|---|---|---|---|---|---|
|
Ripertamab Repatumab |
CD20 | CD20 inhibitor | Monoclonal antibodies | Diffuse large B-cell lymphoma; non-Hodgkin's lymphoma | Sinocelltech Group Ltd. | Approval for listing |
| Mosunetuzumab | CD20 | CD3 | CD20 inhibitor | CD3 inhibitor | Bispecific antibodies | Follicular lymphoma; diffuse large B-cell lymphoma; B-cell lymphoma; non-Hodgkin's lymphoma; B-cell chronic lymphocytic leukemia | Roche (China) Investment Ltd; Roche Registration GmbH | Approval for listing |
|
Ocrelizumab Ocrelizumab |
CD20 | CD20 inhibitor | Monoclonal antibodies | Multiple sclerosis; chronic progressive multiple sclerosis; relapsing-remitting multiple sclerosis; Hashimoto encephalitis | F. Hoffmann-La Roche Ltd.; Genentech, Inc.; Roche Holding AG | Approval for listing |
|
Rituximab/Hyaluronidase Rituximab/Hyaluronidase |
CD20 | Hyaluronic acid | CD20 inhibitor | Monoclonal Antibodies | Enzymes | B-cell chronic lymphocytic leukemia; diffuse large B-cell lymphoma; follicular lymphoma | Roche Holding AG; Genentech, Inc.; Halozyme Therapeutics, Inc. | Approval for listing |
|
Obinutuzumab Otuzumab |
CD20 | CD20 inhibitor | Monoclonal antibodies | Follicular lymphoma; non-Hodgkin's lymphoma; B-cell chronic lymphocytic leukemia; marginal zone B-cell lymphoma; systemic lupus erythematosus; nephropathy; membranous glomerulonephritis; glomerulonephritis; lupus nephritis; diffuse large B-cell lymphoma; colorectal cancer; macroglobulinemia; B-cell lymphoma; lymphoplasmacytic lymphoma; graft-versus-host disease; central nervous system tumors; set of cells lymphoma; lymphoma; renal cell carcinoma; solid tumor; nephrotic syndrome | Shanghai Roche Pharmaceutical Co., Ltd; Genentech, Inc.; Biogen, Inc.; Chugai Pharmaceutical Co. | Approval for listing |
|
Ofatumumab Ofatumumab |
CD20 | CD20 inhibitor | Monoclonal antibodies | Relapsing-remitting multiple sclerosis; multiple sclerosis; lymphocytic leukemia; B-cell chronic lymphocytic leukemia | Novartis Pharma AG; GSK Plc; Novartis AG; Novartis Pharmaceuticals Corp.; Genmab A/S; Novartis Ireland Ltd. | Approval for listing |
|
Ibritumomab Tiuxetan Tiimomab |
CD20 | CD20 inhibitor | Therapeutic radiopharmaceuticals | Radiolabeled antibodies | Monoclonal antibodies | Set of cell lymphoma; non-Hodgkin's lymphoma | Acrotech Biopharma LLC; CASI Pharmaceuticals, Inc.; Spectrum Pharmaceuticals, Inc.; Mundipharma KK | Approval for listing |
|
Rituximab Rituximab |
CD20 | CD20 inhibitor | Monoclonal antibodies | lymphocytic leukemia; thrombocytopenic purpura; renal transplant rejection; liver transplant rejection; nephrotic syndrome; vasculitis; polyarteritis nodosa; sarcoidosis with polyangiitis; microscopic polyangiitis; B-cell chronic lymphocytic leukemia; rheumatoid arthritis; diffuse large B-cell lymphoma; aspergillosis; systemic scleroderma; follicular lymphoma; B-cell lymphoma; non-Hodgkin lymphoma; Burkitt's lymphoma; Hodgkin's lymphoma; multiple myeloma | Roche Holding AG; Shanghai Roche Pharmaceuticals Ltd.; IDEC Pharmaceuticals Corp.; Chugai Pharmaceutical Co., Ltd.; Genentech, Inc.; Cipla Ltd; Roche Pharma (Schweiz) AG | Approval for listing |
| 304 (3SBio) | CD20 | CD20 inhibitor | Monoclonal antibodies | Non-Hodgkin's lymphoma | Samson Pharmaceutical Group | Application for listing |
| Epcoritamab | CD20 | CD3 | CD20-directed cytolysis | CD3 inhibitor | CD20 inhibitor | Bispecific antibodies | Diffuse large B-cell lymphoma; large B-cell lymphoma; follicular lymphoma; hematologic neoplasm | AbbVie, Inc.; AbbVie Pharmaceutical Trading (Shanghai) Co. | Application for listing |
| Glofitamab | CD20 | CD3 | Antibody-dependent cytotoxicity | CD3 inhibitor | CD20 inhibitor | Bispecific antibodies | Diffuse large B-cell lymphoma; follicular lymphoma; non-Hodgkin's lymphoma; lymphoma; B-cell lymphoma | Roche Holding AG; F. Hoffmann-La Roche Ltd.; Roche (China) Investment Ltd.; Genentech, Inc. | Application for listing |
| Ublituximab | CD20 | CD20 inhibitor | Monoclonal antibodies | Relapsing-remitting multiple sclerosis; multiple sclerosis; diffuse large B-cell lymphoma; follicular lymphoma; set of cell lymphoma; marginal zone B-cell lymphoma; B-cell chronic lymphocytic leukemia; non-Hodgkin's lymphoma; optic neuromyelitis optica; autoimmune disease; tumor | TG Therapeutics, Inc.; Ildong Pharmaceutical Co. | Application for listing |
|
Zebituzumab Zebituzumab |
CD20 | CD20 inhibitor | Monoclonal antibodies | Non-Hodgkin's lymphoma; thrombocytopenia; idiopathic thrombocytopenic purpura | Zhejiang Borui Biopharmaceutical Co. | Application for listing |
| Divozilimab | CD20 | CD20 inhibitor | Monoclonal antibodies | Multiple Sclerosis | Biocad CJSC | Clinical Phase 3 |
| MB-CART2019.1 | CD20 | CD19 | T-lymphocyte replacement | CD19 inhibitor | CD20 inhibitor | CAR-T | Non-Hodgkin's lymphoma; diffuse large B-cell lymphoma; B-cell chronic lymphocytic leukemia | Miltenyi Biomedicine GmbH; Miltenyi GmbH; Miltenyi Biotec, Inc. | Clinical Phase 3 |
|
Recombinant chimeric anti-CD20 antibody(Shanghai Institute of Biological Products Co., Ltd.) Recombinant chimeric anti-CD20 monoclonal antibody (Shanghai Institute of Biological Products) |
CD20 | CD20 inhibitor | Monoclonal antibodies | Diffuse large B-cell lymphoma | Shanghai Institute of Biological Products Co. | Clinical Phase 3 |
|
Recombinant humanized monoclonal antibody MIL62 Recombinant humanized monoclonal antibody MIL62 |
CD20 | CD20 inhibitor | Monoclonal antibodies | Follicular lymphoma; marginal zone B-cell lymphoma; B-cell lymphoma; systemic lupus erythematosus; myasthenia gravis; non-Hodgkin's lymphoma; optic neuromyelitis optica | Jiangsu Hengrui Pharmaceutical Co., Ltd; Beijing Tianguang Shi Biotechnology Co. | Clinical Phase 3 |
| 4SCAR-T cell therapy (Shenzhen Geno-Immune Medical Institute) | CD20 | CD22 | CD38 | CD20 inhibitor | CD22 modulator | CD38 inhibitor | CAR-T | Tumors | Shenzhen Institute of Immunogene Therapy | Clinical Phase 2 |
| Anti-CD19 and anti-CD20 CAR-T cell therapy (The Medical College of Wisconsin, Inc.) | CD20 | CD19 | T-lymphocyte replacement | CD19 inhibitor | CD20 inhibitor | CAR-T | Tumors | The Medical College of Wisconsin, Inc. | Clinical Phase 2 |
| Anti-CD20 CAR T-cell therapy(Shanghai Longyao Biotech Co., Ltd.) | CD20 | CD20 inhibitor | CAR-T | Diffuse large B-cell lymphoma | Shanghai Longyao Biotech Co., Ltd. | Clinical Phase 2 |
|
Anti-CD20 CART-transduced T cells (Chinese People's Liberation Army General Hospital) Anti-CD20 CART-transduced T cells (Chinese People's Liberation Army General Hospital) |
CD20 | CD20 inhibitor | CAR-T | B-cell lymphoma; leukemia; non-Hodgkin's lymphoma | Cellular Biomedicine Group, Inc.; General Hospital of the Chinese People's Liberation Army | Clinical Phase 2 |
| Autologous Anti-CD19/CD20 CAR T-cell Therapy (Kite Pharma, Inc) | CD20 | CD19 | T-lymphocyte replacement | CD19 inhibitor | CD20 inhibitor | CAR-T | Tumor; B-cell lymphoma; diffuse large B-cell lymphoma | Kite Pharma, Inc. | Clinical Phase 2 |
| BVX20-CD20 antibody(Biocon Ltd.) | CD20 | CD20 inhibitor | Monoclonal antibodies | Non-Hodgkin's lymphoma; multiple sclerosis | Vaccinex, Inc.; Biocon Ltd. | Clinical Phase 2 |
| CD20 monoclonal antibody(The First Affiliated Hospital of Soochow University) | CD20 | CD20 modulator | Monoclonal antibodies | Aplastic anemia | The First Hospital of Soochow University | Clinical Phase 2 |
| CPO-107 | CD20 | CD47 | CD47 inhibitor | CD20 inhibitor | Fusion proteins | Non-Hodgkin's lymphoma | Conjupro Bioerapecitics, Inc. | Clinical Phase 2 |
| DI-Leu16-IL2 | CD20 | IL2R | CD20 inhibitor | IL2R agonist | Fusion proteins | Non-Hodgkin's lymphoma | Alopexx Oncology, LLC; Merck Serono SA; Provenance Biopharmaceuticals Corp. | Clinical Phase 2 |
| MRG001 | CD20 | Tubulin | CD20 inhibitor | ADC | Monoclonal Antibodies | Novel coronavirus pneumonia; adult respiratory distress syndrome; non-Hodgkin's lymphoma | Lepu Biopharma Co., Ltd.; MedRegen LLC; Shanghai Mayak Biotechnology Co. | Clinical Phase 2 |
| MT-3724 | CD20 | CD20 inhibitor | Antibody-coupled toxins | B-cell chronic lymphocytic leukemia; B-cell lymphoma; diffuse large B-cell lymphoma; hematologic neoplasm | Molecular Templates, Inc. | Clinical Phase 2 |
| Odronextamab | CD20 | CD3 | CD20-directed cytolysis | CD3 inhibitor | CD20 inhibitor | Bispecific antibodies | Non-Hodgkin's lymphoma; Follicular lymphoma; Lymphoma; B-cell chronic lymphocytic leukemia; B-cell lymphoma; Lymphocytic leukemia | Regeneron Pharmaceuticals, Inc.; Redding Pharmaceuticals (Shanghai) Co. | Clinical Phase 2 |
|
Plamotamab Paramotumab |
CD20 | CD3 | CD20-directed cytolysis | CD3 inhibitor | CD20 inhibitor | Bispecific Antibodies | Monoclonal Antibodies | Primary generalized cutaneous diffuse large B-cell lymphoma; diffuse large B-cell lymphoma; B-cell chronic lymphocytic leukemia; non-Hodgkin's lymphoma; B-cell lymphoma | Xencor, Inc.; Novartis Pharma AG | Clinical Phase 2 |
| Veltuzumab | CD20 | CD20 inhibitor | Monoclonal antibodies | Non-Hodgkin's lymphoma; idiopathic thrombocytopenic purpura | Immunomedics, Inc. | Clinical Phase 2 |
| 4SCAR20 (Shenzhen Geno-Immune Medical Institute) | CD20 | CD20 inhibitor | CAR-T | B-cell leukemia; B-cell lymphoma; tumor; primary central nervous system lymphoma; drug-resistant cancer | Shenzhen Institute of Immunogene Therapy | Clinical Phase 1/2 |
| Anti-CD19/CD20 CAR-T cell therapy (Shanghai Longyao Biotechnology) | CD20 | CD19 | T-lymphocyte replacement | CD20 inhibitor | CD19 inhibitor | CAR-T | B-cell lymphoma | Shanghai Longyao Biotech Co., Ltd.; Xuzhou Medical University; Shanghai Jiao Tong University | Clinical Phase 1/2 |
| CD20-targeted CAR T cell therapy (Mustang Bio) | CD20 | CD20 inhibitor | CAR-T |
B-cell chronic lymphocytic leukemia; refractory B-cell lymphoma; follicular lymphoma; relapsed set of cell lymphoma; refractory set of cell lymphoma; small lymphocytic lymphoma; relapsed macroglobulinemia; refractory Walden's macroglobulinemia Non-Hodgkin's lymphoma |
Mustang Bio, Inc.; Fred Hutchinson Cancer Research Center | Clinical Phase 1/2 |
| CMG1A46 | CD20 | CD3 | CD19 | CD3 modulators | CD19 inhibitors | CD20 inhibitors | Tri-Specific Antibodies | Acute lymphoblastic leukemia; non-Hodgkin's lymphoma; tumor | Chengdu Enmu Biotechnology Co., Ltd; Zhejiang Borui Bio Pharmaceutical Co. | Clinical Phase 1/2 |
| IMM-0306 | CD20 | CD47 | CD47 inhibitor | CD20 inhibitor | Bispecific antibodies | B-cell lymphoma; non-Hodgkin's lymphoma | Yiming Onco Biomedical Technology (Shanghai) Co. | Clinical Phase 1/2 |
| JMT-601 | CD20 | SIRPA | SIRPA inhibitor | CD20 inhibitor | Fusion Proteins | Bispecific Antibodies | Non-Hodgkin's lymphoma | Conjupro Biotherapeutics, Inc.; Shanghai Zimantec Biotechnology Co. | Clinical Phase 1/2 |
| Tandem CAR19/20 engineered T cells (Chinese PLA General Hospital) | CD20 | CD19 | CD20 inhibitor | CD19 inhibitor | CAR-T | Attack; B-cell lymphoma; lymphoma; tumor; non-Hodgkin's lymphoma | General Hospital of the Chinese People's Liberation Army | Clinical Phase 1/2 |
| UCART 20x22 | CD20 | CD22 | T-lymphocyte replacement | CD20 inhibitor | CD22 inhibitor | CAR-T | Non-Hodgkin's lymphoma; precursor B-cell adult lymphocytic leukemia lymphoma | Cellectis SA | Clinical Phase 1/2 |
| ACE-1831 | CD20 | T-lymphocyte replacement | CD20 inhibitor | Gene Therapy | Non-Hodgkin's lymphoma; hematologic neoplasm | Acepodia Biotech, Inc. | Clinical Phase 1 |
| Anti-CD19 anti-CD20 CAR-T cell therapy (PersonGen BioTherapeutics) | CD20 | CD19 | T-lymphocyte replacement | CD20 inhibitor | CD19 inhibitor | CAR-T | Hematologic tumors; non-Hodgkin's lymphoma | Boshengji Pharmaceutical Technology (Suzhou) Co. | Clinical Phase 1 |
| Anti-CD19/CD20/CD22 CAR T-Cells (Ohio State University Comprehensive Cancer Centerc) | CD20 | CD19 | CD22 | T-lymphocyte replacement | CD19 inhibitor | CD20 inhibitor | CD22 inhibitor | CAR-T | Acute lymphoblastic leukemia; B-cell lymphoblastic leukemia; high-grade B-cell lymphoma; relapsed B acute lymphoblastic leukemia; relapsed inert non-Hodgkin's lymphoma; relapsed non-Hodgkin's lymphoma; relapsed transformed chronic lymphocytic leukemia; refractory B acute lymphoblastic leukemia; refractory pre-B-cell lymphocytic leukemia; refractory inert non-Hodgkin's lymphoma. Refractory transformed chronic lymphocytic leukemia | Ohio State University Comprehensive Cancer Center | Clinical Phase 1 |
| Anti-CD20 B9E9 scFv-Streptavidin Fusion Protein (Fred Hutchinson Cancer Research Center) | CD20 | CD20 inhibitor | Fusion proteins | Burkitt's lymphoma; diffuse large B-cell lymphoma; set cell lymphoma; non-Hodgkin's lymphoma | Fred Hutchinson Cancer Research Center; National Cancer Institute | Clinical Phase 1 |
| Anti-CD20/CD22 CAR-T cell therapy (Yake Biotechnology) | CD20 | CD22 | T-lymphocyte replacement | CD20 inhibitor | CD22 inhibitor | CAR-T | Hematologic Tumors | Shanghai Artech Biotechnology Co. | Clinical Phase 1 |
| B-001 | CD20 | CD20 inhibitor | Monoclonal antibodies | Non-Hodgkin's lymphoma; B-cell chronic lymphocytic leukemia | Shanghai Pharmaceutical Group Co. | Clinical Phase 1 |
| BAT-4406F | CD20 | CD20 inhibitor | Monoclonal antibodies | Optic neuromyelitis optica; multiple sclerosis | Biotest Biopharmaceutical Co. | Clinical Phase 1 |
| C-CAR039 | CD20 | CD19 | CD20 inhibitor | CD19 inhibitor | Autologous CAR-T | Non-Hodgkin's lymphoma | Cellular Biomedicine Group, Inc. | Clinical Phase 1 |
| CD 20 CAR-T cell therapy (Shanghai Longyao Biotechnology) | CD20 | T-lymphocyte replacement | CD20 inhibitor | CAR-T | Non-Hodgkin's lymphoma | Shanghai Longyao Biotech Co., Ltd. | Clinical Phase 1 |
|
CD19/CD20 bispecific chimeric antigen receptor (CAR)-T cell therapy(Shanghai Cellular Biopharmaceutical Group) Anti-CD19/CD20 chimeric antigen receptor autologous T cells (Shanghai Cellular Biopharmaceutical Group) |
CD20 | CD19 | CD20 inhibitor | CD19 inhibitor | CAR-T | Large B-cell lymphoma | Shanghai Saibiman Biotechnology Co. | Clinical Phase 1 |
|
CD19/CD20 bispecific chimeric antigen receptor (CAR)-T cell therapy (University of California) CD19/CD20 bispecific chimeric antigen receptor (CAR)-T cell therapy (University of California) |
CD20 | CD19 | T-lymphocyte replacement | CD20 inhibitor | CD19 inhibitor | CAR-T | B-cell chronic lymphocytic leukemia; B-cell lymphoma | University of California | Clinical Phase 1 |
Table 1. Data source: Synapse
To fully support researchers and pharmaceutical companies in their research on CD20 for the treatment of lymphoma, leukemia, autoimmune diseases, CUSABIO boasts CD20 active protein products (Code: CSB-MP015007HU) to assist you in your research on the mechanism of CD20 or its potential clinical value (Click to see a broad range of CD20-based products: Proteins ; Antibodies ; ELISAs).
Recombinant Human B-lymphocyte antigen CD20(MS4A1)-VLPs (Active)
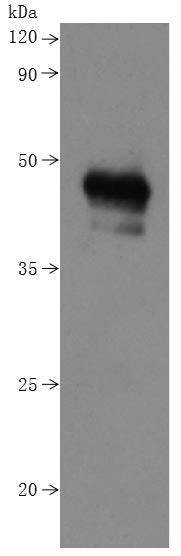
CSB-MP015007HU is detected by Mouse anti-6*His monoclonal antibody.
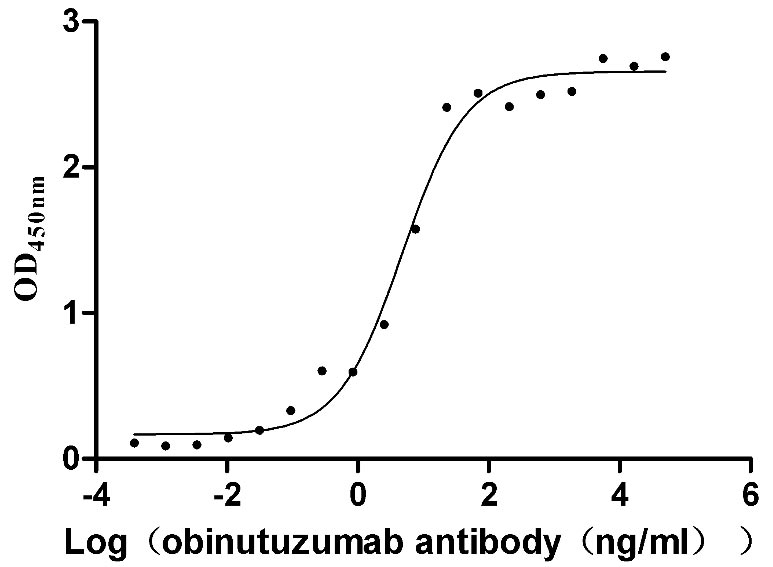
Immobilized human CD20 at 2 μg/ml can bind Anti-CD20 recombinant antibody (CSB-RA015007A1HU), the EC50 is 3.243-7.085 ng/mL.
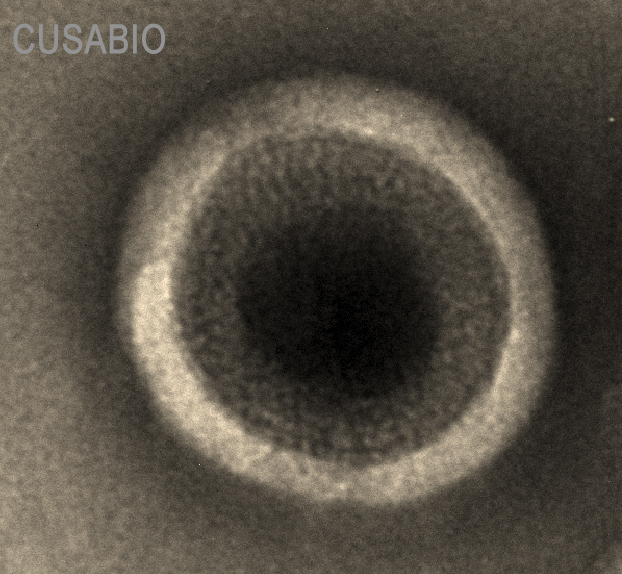
The presence of VLP-like structures was confirmed by TEM
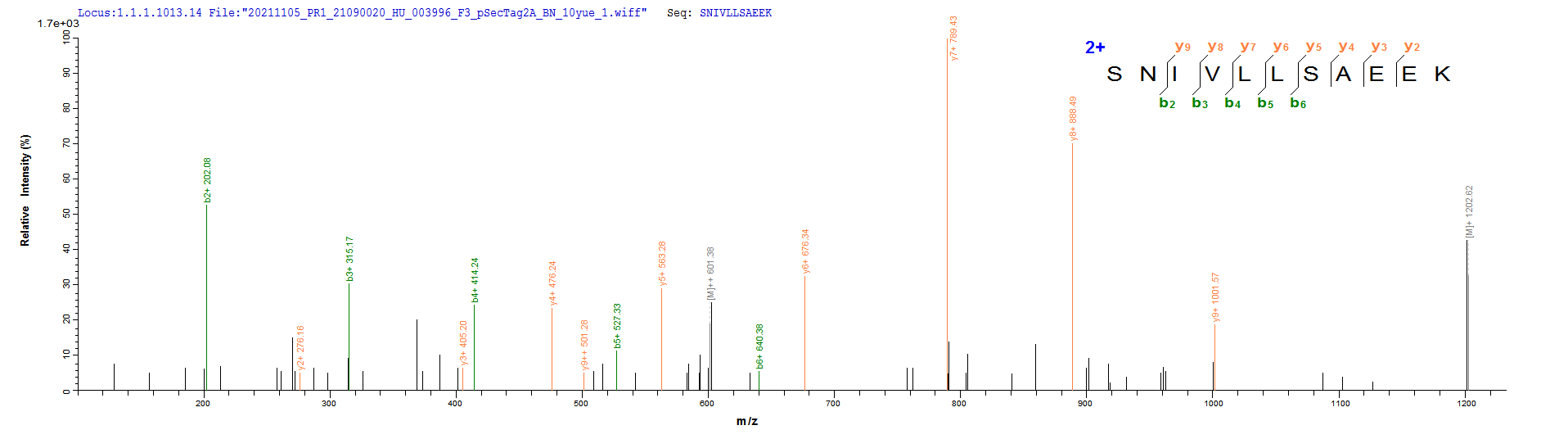
Based on the SEQUEST from database of Mammalian Cell host and target protein, the LC-MS/MS Analysis result of CSB-MP015007HU could indicate that this peptide derived from Mammalian Cell-expressed Homo sapiens (Human) CD20.
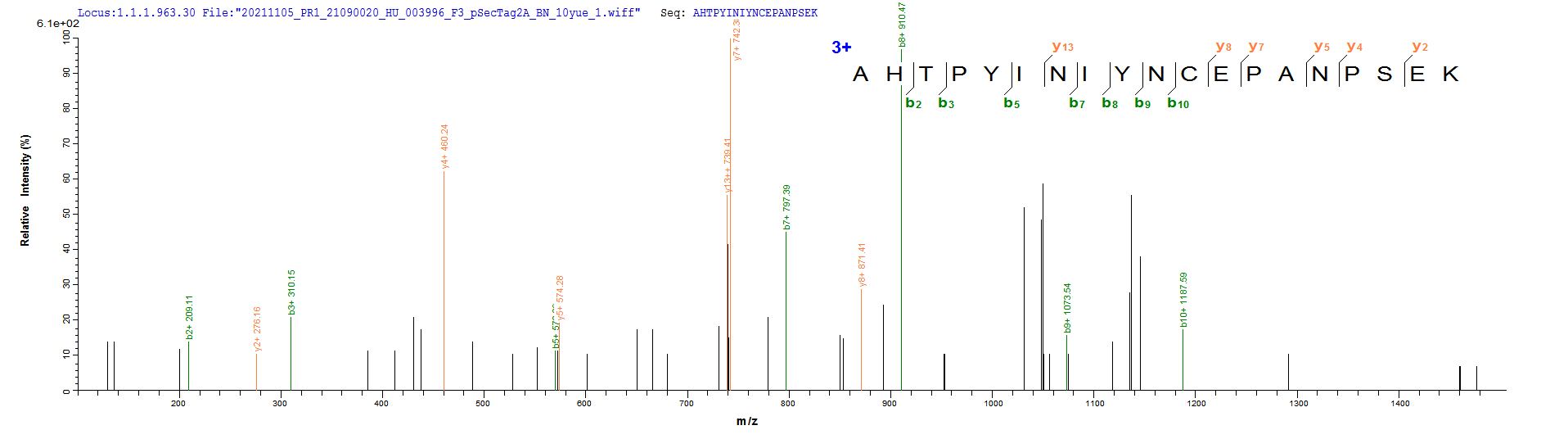
Based on the SEQUEST from database of Mammalian Cell host and target protein, the LC-MS/MS Analysis result of CSB-MP015007HU could indicate that this peptide derived from Mammalian Cell-expressed Homo sapiens (Human) CD20.
CD20 ( MS4A1) Antibodies
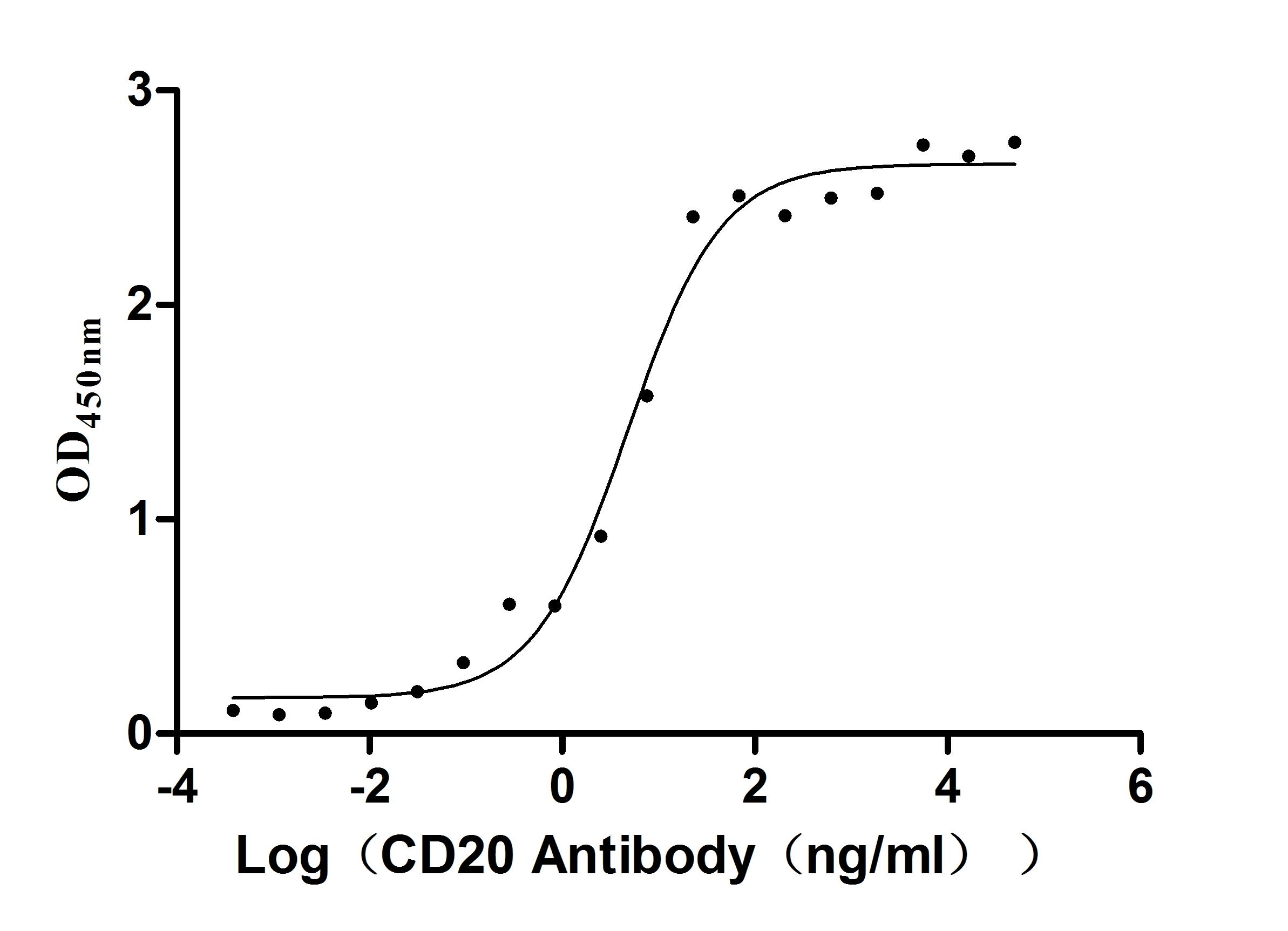
MS4A1 Monoclonal Antibody
(CSB-RA015007A1HU)
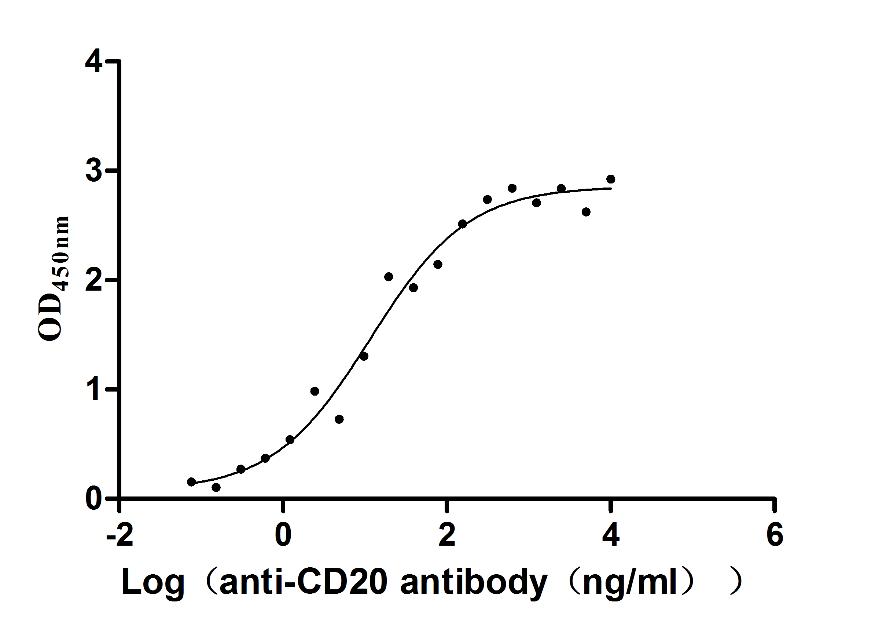
MS4A1 Monoclonal Antibody
(CSB-RA015007MA3HU)
References
[1] Shi, Yuankai, et al. "Comparison of efficacy and safety of ripertamab (SCT400) versus rituximab (Mabthera®) in combination with CHOP in patients with previously untreated CD20-positive diffuse large B-cell lymphoma: A randomized, single-blind, phase III study. previously untreated CD20-positive diffuse large B-cell lymphoma: A randomized, single-blind, phase III clinical trial." Hematological Oncology (2022).
[2] Wu, Xin, et al. "The Efficacy and Safety of Anti-CD20 Antibody Treatments in Relapsing Multiple Sclerosis: A Systematic Review and Network Meta- analysis." CNS drugs (2022): 1-16.
[3] Schilhabel, Anke, et al. "CD20 Expression as a Possible Novel Prognostic Marker in CLL: Application of EuroFlow Standardization Technique and Normalization Procedures in Flow Cytometric Expression Analysis." Cancers 14.19 (2022): 4917.
[4] Pavlasova, Gabriela, and Marek Mraz. "The regulation and function of CD20: an "enigma" of B-cell biology and targeted therapy." Haematologica 105.6 (2020): 1494.
[5] Shree, Tanaya, et al. "CD20-targeted therapy ablates de novo antibody response to vaccination but spares preestablished immunity." Blood cancer discovery 3.2 (2022): 95-102.
[6] Cree, Bruce AC. "All anti-CD20 monoclonal antibodies have similar efficacy and safety risks: Yes." Multiple Sclerosis Journal 28.12 (2022): 1843-1844.
[7] Schuster, Stephen J., et al. "Characterization of CD20 expression loss as a mechanism of resistance to mosunetuzumab in patients with relapsed/ refractory B-cell non-Hodgkin lymphomas."(2022): 7526-7526.
[8] Walshe, Claire A., et al. "Induction of cytosolic calcium flux by CD20 is dependent upon B Cell antigen receptor signaling." Journal of Biological Chemistry 283.25 (2008): 16971-16984.
[9] Liu, Jinny L., et al. "Selection and characterization of single domain antibodies against human CD20." Molecular Immunology 78 (2016): 146-154.
[10] Schneider, Dina, et al. "Trispecific CD19-CD20-CD22-targeting duoCAR-T cells eliminate antigen-heterogeneous B cell tumors in preclinical models." Science Translational Medicine 13.586 (2021): eabc6401.
[11] Grandjean, Capucine L., et al. "Imaging the mechanisms of anti-CD20 therapy in vivo uncovers spatiotemporal bottlenecks in antibody-dependent phagocytosis." Science Advances 7.8 (2021): eabd6167.
[12] Pozzo, Federico, et al. "SF3B1-mutated chronic lymphocytic leukemia shows evidence of NOTCH1 pathway activation including CD20 downregulation." Haematologica 106.12 (2021): 3125.
[13] Goldenberg, David M., and Robert M. Sharkey. "Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy." Expert Opinion on Biological Therapy 20.8 (2020): 871-885.
[14] Asano, Teizo, et al. "Epitope mapping of an anti-CD20 monoclonal antibody (C20Mab-60) using the HisMAP method." Monoclonal antibodies in immunodiagnosis and immunotherapy 40.6 (2021): 243-249.
[15] Luo, Chengxin, et al. "Efficacy and safety of new anti-CD20 monoclonal antibodies versus rituximab for induction therapy of CD20+ B-cell non-Hodgkin lymphomas: A systematic review and meta-analysis." Scientific reports 11.1 (2021): 1-14.
Comments
Leave a Comment