Presently, a paper entitled "TGF-β induces GBM mesenchymal transition through upregulation of CLDN4 and nuclear translocation to activate TNF-α/NF-κB signal pathway" was published on Cell Death & Disease. The study firstly reported that CLDN4 was remarkably upregulated in glioblastoma specimens and cells. Beyond this, the researchers based on in vitro analysis found that CLDN4 regulates TNF-α signal pathway. Further experimental data showed that CLDN4 knockdown inhibited the growth and invasion in the xenograft mouse model. Moreover, CLDN4 combined with ITD-1 (TGF-β signal pathway inhibitor) achieve better results [1]. CLDN4, an important molecule of the Claudins family. Successive studies have shown that CLDN4 functions as a specific target for tumor treatment. Encouragingly, more papers have identified that the combination of anti-CLDN4 antibodies in tumor treatment can strengthen the anti-tumor effect. Therefore, following CLDN18.2, and CLDN9, and CLDN6, CLDN4 is expected to be an effective target for tumor-targeted therapy and holds great potential for drug combination therapy.
1. What is CLDN4?
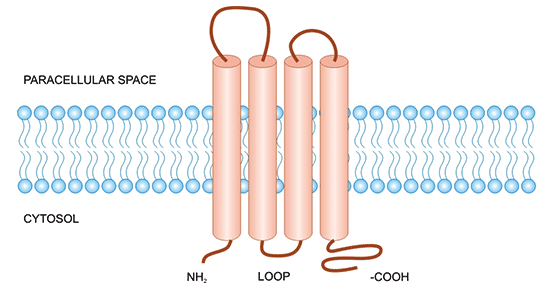
Claudin 4/CLDN4 is a member of the Claudins family and is one of the important junctional proteins of Tight Junctions (TJ). Claudins are transmembrane proteins with domains 1 to 4, with the N-terminal and C-terminal located inside the cell, and a binding domain at the C-terminus that binds to proteins in the inner cytoplasm (e.g., ZO-1), which play an important role in signal transduction. The extracellular membrane has two extracellular loops ECL1 and ECL2 (Figure 1) [2]. The extracellular loops are required to maintain tight junction function and epithelial barrier integrity.
Claudins are tight junction membrane proteins that are expressed in epithelia and endothelia and form paracellular barriers and pores that determine tight junction permeability. Any alteration or disruption in claudins could cause disorder of epithelial cell and polarity, and then defects in junction formation or maintenance, which in turn leads to various pathophysiological conditions like cancer [2, 3]. More and more studies revealed that CLDN4 plays a regulatory role in cancer and may be a potential tumor therapeutic target [4-6]. In fact, a large body of evidence has shown that Claudins play an important role in tumor immunotherapy and boasts a great potential for drug research (See more previous articles about Claudins family CLDN6; CLDN9; CLDN18.2).
Figure 1. The structure of CLDN4 [2]
2. How's the Mechanism of CLDN4?
Related studies indicate that Claudins are involved in tumors as follow: 1) aberrant expression of Claudins causes tight junction disorder and leakage, which promotes metastasis and invasion of tumor cells; 2) decreased cell polarity increases the supply of nutrients and growth factors, which accelerates the proliferation of tumor cells; 3) decreased intercellular adhesion increases the risk of metastasis and promotes the tumor development [7-9]. Currently, CLDN4 has been found widely aberrantly expressed in tumors, but its mechanisms in tumors remain to be fully understood.
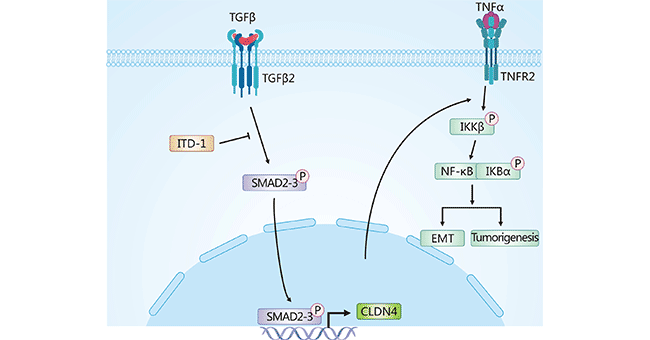
In glioma, it was found that the CLDN4 induces EMT in glioma by regulating the TNF-α mediated NF-κB signaling pathway (Figure 2) [1]. In ovarian cancer, upregulated CLDN4 can mediate PI3K/Akt and EMT transcription factor Twist1 [10, 11]. In pancreatic cancer, TGF-β can negatively mediate CLDN4 expression, and overexpression of CLDN4 inhibits invasion of pancreatic cancer cells [12]. In breast cancer, CLDN4 promotes tumor cell migration and invasion through the PAK4-CEBPB-CLDN4 axis [13].
In addition, CLDN4 was lowly expressed in human laryngeal carcinoma Hep-2 cells. It has been demonstrated that CLDN4 inhibits cell proliferation through JAK2/STAT3 signaling pathway [14]. Another study concluded that in laryngeal squamous carcinoma Hep-2 cells, DNA demethylation of CLDN4 suppressed migration and invasion of HEp-2 cells, which was associated with MeCP2 recruitment HDAC1. It was further found that CLDN4 overexpression inhibited Hep-2 cell proliferation and increased the number of intercellular tight junctions [15].
Figure 2. CLDN4 induced EMT pathway in glioma cells [1]
3. The Functional Roles of CLDN4 in Cancers
CLDN4 is highly expressed or deficiently expressed in a variety of tumors. Especially in gastrointestinal cancer, including esophageal tumors, gastric cancer, colon cancer, liver cancer, pancreatic cancer, laryngeal squamous cell carcinoma, etc.; and in gynecologic cancer, such as ovarian cancer and cervical cancer, there are studies showing abnormally high or low expression of CLDN4 in these tumor tissues. Therefore, CLDN4 might be used as a promising biomarker in tumors for early diagnosis, efficacy evaluation, prognosis prediction, even in clinical drug research.
3.1 CLDN4 and Gastrointestinal Cancer
In pancreatic cancer, it has been shown that Rosiglitazone induced the CLDN4 expression via inhibiting MEK-ERK signaling pathway. Upregulated CLDN4 expression curbed pancreatic cancer cell invasion, suggesting that CLDN4 is a potential inhibitor of pancreatic cancer cells [17]. Whereas cDNA microarrays indicated that CLDN4 may be involved in the development of early pancreatic cancer [18]. In gastric cancer, CLDN4 was aberrantly highly expressed. The transfection of CLDN4 siRNA in gastric cancer cell line SGC7901 suggested a significant decrease in the cell invasive. Furthermore, knockdown of CLDN4 enhanced the phosphorylation of PI3K and AKT, promoting the cell proliferation, migration, and invasion, as well as reducing the sensitivity of gastric cancer cells to chemotherapy [19, 20].
In hepatocellular carcinoma, zinc finger protein 703 (ZNF703) binding to the CLDN4 promoter activates CLDN4, and then inducing EMT process, which thereby strengthen the tumor cell metastasis and sorafenib resistance [21]. In laryngeal squamous cell carcinoma, CLDN4 expression was downregulated. Studies confirmed that CLDN4 in laryngeal cancer tissues was correlated with MECP-2 expression. Further studies implied that DNA methylation transferase inhibitor (5-aza-dc) acting on HEP-2 cells upregulated CLDN4 expression and impeded the cell migration and invasive [22]. Overall, CLDN4 may be a molecular marker for the diagnosis of gastrointestinal tumors.
3.2 CLDN4 and Gynecologic Cancer
In ovarian cancer, overexpression of CLDN4 stimulates the EMT process. High expression of CLDN4 is associated with poor patient prognosis [10, 11]. Recently, studies identified that CLDN4 deletion inhibits DNA repair and increases sensitivity to the PARP inhibitor. In contrast, CLDN4 overexpression decreased sensitivity to the PARP inhibitor [23]. In cervical cancer, CLDN4 expression was significantly upregulated in cervical cancer tissues compared with normal squamous epithelium. As precancerous developing a more severe condition, the level of CLDN4 expression increased. Thus, CLDN4 expression might be partly correlated with the development of cervical cancer [24]. In addition, high-risk human papillomavirus (HR-HPV) combined with CLDN4 testing can improve the specificity of cervical precancer diagnosis. HR-HPV has been shown to be the key contributor to the development of HPV-induced cervical cancer. Therefore, CLDN4 might serve as an important factor for the diagnosis of ovarian cancer and cervical precancerous lesions.
3.3 CLDN4 and Other Cancers
CLDN4 is highly sensitive to CPE in vitro. Clostridium perfringens enterotoxin (CPE), an important causative agent of food poisoning and gastrointestinal diseases in humans. In prostate cancer, direct injection of CPE into tumor cells reduced tumor volume significantly. CLDN3 and CLDN4 act as receptors for CPE. CPE specifically binds to the receptor and causes cell lysis and necrosis through cytosolic penetration. Studies have shown that the cytotoxicity of CPE is restricted to cancer cells with high expression of CLDN3 and CLDN4 [25--27]. As an alternative approach, the integration of CPE treatment could be applied to obtain potent killing properties against tumors expressing claudin receptors for CPE.
In gliomas, Kaplan-Meier analysis based on the TCGA database showed that CLDN4 expression was negatively correlated with OS and DFS. Moreover, CLDN4 was found to regulate TNF-α induced NF-κB activity. Blocking the CLDN4/TNFα/NF-κB signaling axis may be a novel strategy for glioma treatment [1]. In triple negative breast cancer, CLDN4 expression is higher than normal group. The use of anti-CLDN4 antibody (4D3) impairs tight junction function and enhances the anti-tumor effect of Paclitaxel [28]. In bladder cancer, combination with cisplatin therapy, 4D3 enhanced cisplatin cytotoxicity by increasing cellular permeability, leading to increased intracellular cisplatin concentrations [29].
4. Clinical Research Prospects of CLDN4
Claudins are important molecules that constitutes cell-cell TJs, which have crucial roles in regulating paracellular permeability and maintaining cell polarity. CLDN proteins with aberrant expression can play a cancer-promoting or tumor suppressor role in tumorigenesis development. Currently, there are increasing new insights into the role of CLDN4, suggesting that abnormal expression of CLDN4 can affect the invasive and metastatic ability of tumors. Especially in tumors with high CLDN4 expression, CLDN4 is expected to serve as a tumor marker and therapeutic target. Increasing evidence demonstrated that combination anti-CLDN4 antibody therapy has potential applications to enhance the effects of drugs. With more focus on claudins family, it is believed that more CLDN protein will provide new ideas for the diagnosis and treatment of a variety of diseases.
To fully support researchers and pharmaceutical companies in their research on the CLDN4 targeted drug research in tumors, CUSABIO presents CLDN4 active protein (Code: CSB-MP005506HU) to assist you in your research on the mechanism of CLDN4 or its potential clinical value.
References
[1] Yan, Tengfeng, et al. "TGF-β induces GBM mesenchymal transition through upregulation of CLDN4 and nuclear translocation to activate TNF-α/NF-κB signal pathway." Cell death & disease 13.4 (2022): 1-11.
[2] Uthayanan, Leshanth, and Mona El-Bahrawy. "Potential roles of claudin-3 and claudin-4 in ovarian cancer management. "Journal of the Egyptian National Cancer Institute 34.1 (2022): 1-9.
[3] Yao, Qing-Ting, et al. "Pilocarpine improves submandibular gland dysfunction in irradiated rats by downregulating the tight junction protein claudin-4." Oral Diseases 28.6 (2022): 1528-1538.
[4] Liu, Hong, et al. "Claudin-1/4 as directly target gene of HIF-1α can feedback regulating HIF-1α by PI3K-AKT-mTOR and impact the proliferation of esophageal squamous cell though Rho GTPase and p-JNK pathway." Cancer Gene Therapy 29.6 (2022): 665-682.
[5] Maesaka, Fumisato, et al. "Hypomethylation of CLDN4 Gene Promoter Is Associated with Malignant Phenotype in Urinary Bladder Cancer." International Journal of Molecular Sciences 23.12 (2022): 6516.
[6] Kim, Won Shik, et al. "High Expression of Claudin-4 Is Associated with Synchronous Tumors in Patients with Early Gastric Cancer." journal of clinical medicine 11.12 (2022): 3550.
[7] Swisshelm, Karen, Robert Macek, and Manfred Kubbies. "Role of claudins in tumorigenesis." advanced drug delivery reviews 57.6 (2005): 919-928.
[8] Kage, Hidenori, et al. "Dichotomous roles of claudins as tumor promoters or suppressors: lessons from knockout mice." Cellular and Molecular Life Sciences 76.23 (2019): 4663-4672.
[9] Li, Jian. "Context-dependent roles of claudins in tumorigenesis." Frontiers in oncology 11 (2021): 676781.
[10] Hicks, Douglas A., et al. "Claudin-4 activity in ovarian tumor cell apoptosis resistance and migration." bmc cancer 16.1 (2016): 1-11.
[11] English, Diana P., and Alessandro D. Santin. "Claudins overexpression in ovarian cancer: potential targets for Clostridium Perfringens Enterotoxin ( CPE) based diagnosis and therapy." international journal of molecular sciences 14.5 (2013): 10412-10437.
[12] Michl, Patrick, et al. "Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer." Cancer research 63.19 (2003): 6265-6271.
[13] Wang, Fei, et al. "A novel PAK4-CEBPB-CLDN4 axis involving in breast cancer cell migration and invasion." biochemical and biophysical research communications 511.2 (2019): 404-408.
[14] Shi, Hailian, et al. "SP1 affects the migration and invasion of laryngeal cancer cells by regulating methylation of claudin 4 promoter region through the p-JNK pathway." bioRxiv (2022).
[15] Liu, Yafang, et al. "DNA demethylation of claudin-4 suppresses migration and invasion in laryngeal squamous carcinoma cells." Human Pathology 75 (2018 ): 71-80.
[16] Okumura, Toshikatsu. "Mechanisms by which thiazolidinediones induce anti-cancer effects in cancers in digestive organs." journal of gastroenterology 45.11 (2010): 1097-1102.
[17] Kumei, Shima, et al. "Troglitazone increases expression of E-cadherin and claudin 4 in human pancreatic cancer cells." Biochemical and biophysical research communications 380.3 (2009): 614-619.
[18] Terris B, Blaveri E, Crnogorac-Jurcevic T, et al. Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas[J]. The American journal of pathology, 2002, 160(5): 1745-1754.
[19] Liang Z Y, Kang X, Chen H, et al. Effect of Clostridium perfringens enterotoxin on gastric cancer cells SGC7901 which highly expressed claudin-4 protein[. J]. World Journal of Gastrointestinal Oncology, 2017, 9(4): 153.
[20] Luo, Jie, et al. "CLDN4 silencing promotes proliferation and reduces chemotherapy sensitivity of gastric cancer cells through activation of the PI3K/ Akt signalling pathway." Experimental Physiology 105.6 (2020): 979-988.
[21] Wang, Hao, et al. "Zinc finger protein 703 induces EMT and sorafenib resistance in hepatocellular carcinoma by transactivating CLDN4 expression." Cell death & disease 11.4 (2020): 1-15.
[22] Liu, Yafang, et al. "DNA demethylation of claudin-4 suppresses migration and invasion in laryngeal squamous carcinoma cells." Human Pathology 75 (2018 ): 71-80.
[23] Yamamoto, Tomomi M., et al. "Loss of Claudin-4 Reduces DNA Damage Repair and Increases Sensitivity to PARP Inhibitors." Molecular Cancer Therapeutics 21.4 (2022): 647-657.
[24] Di, Chenhong, and Fan Jin. "Value of combined detection of claudin 4 and high-risk human papilloma virus in high-grade squamous intraepithelial lesion and cervix squamous cell carcinoma." Zhejiang da xue xue bao. yi xue ban Journal of Zhejiang University. medical Sciences 47.4 (2018): 344-350.
[25] Maeda, Toshihiro, et al. "Claudin-4-targeted therapy using Clostridium perfringens enterotoxin for prostate cancer." The Prostate 72.4 (2012): 351-360.
[26] Takahashi, Azusa, et al. "Mutated C-terminal fragments of Clostridium perfringens enterotoxin have increased affinity to claudin-4 and reversibly modulate tight junctions in vitro." Biochemical and biophysical research communications 410.3 (2011): 466-470.
[27] Saeki, Rie, et al. "A novel tumor-targeted therapy using a claudin-4-targeting molecule." Molecular Pharmacology 76.4 (2009): 918-926.
[28] Luo, Yi, et al. "Targeting claudin-4 enhances chemosensitivity in breast cancer." Cancer science 111.5 (2020): 1840-1850.
[29] Kuwada, Masaomi, et al. "Pro-chemotherapeutic effects of antibody against extracellular domain of claudin-4 in bladder cancer." Cancer letters 369.1 (2015): 212-221.
CUSABIO team. CLDN4: A Potential Prognostic Indicator and Therapeutic Target in Tumors!. https://www.cusabio.com/c-21083.html




Comments
Leave a Comment