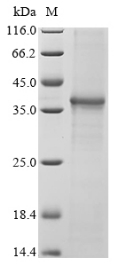
Recombinant human NLRP3 production in E. coli is a multi-step process. First, co-inserting the gene of interest into an expression vector with an N-terminal 10xHis-tag and C-terminal Myc-tag gene. The target gene encodes the 733-1036aa of human NLRP3. And then, transfecting the recombinant vector into E. coli cells. The cells are cultured to produce the NLRP3 protein, which is extracted by cell lysis. Purification of the collected recombinant human NLRP3 protein is done using affinity chromatography. Its purity is assessed using SDS-PAGE, 85%.
The NLRP3 is a key component of the innate immune system involved in inflammatory responses. It is a multi-domain protein comprising an amino-terminal pyrin domain (PYD), a central NACHT domain, and a carboxy-terminal leucine-rich repeat domain (LRR domain) [1][2]. Activation of NLRP3 results in the assembly of the NLRP3-ASC-pro-caspase-1 inflammasome, which activates caspase-1, leading to the release of proinflammatory cytokines IL-1β and IL-18 in response to various stimuli like microbial infections and cellular damage [3][4][5].
NLRP3 functions as a sensor for a broad range of PAMPs and DAMPs, enabling the detection of cellular stress signals induced by infection and sterile inflammation [6][7]. Aberrant NLRP3 activity can cause excessive inflammation, contributing to the development of various chronic diseases [8].
References:
[1] K. Swanson, M. Deng, & J. Ting, The nlrp3 inflammasome: molecular activation and regulation to therapeutics, Nature Reviews Immunology, vol. 19, no. 8, p. 477-489, 2019. https://doi.org/10.1038/s41577-019-0165-0
[2] I. Hochheiser, M. Pilsl, G. Hagelueken, J. Moecking, M. Marleaux, R. Brinkschulteet al., Structure of the nlrp3 decamer bound to the cytokine release inhibitor crid3, Nature, vol. 604, no. 7904, p. 184-189, 2022. https://doi.org/10.1038/s41586-022-04467-w
[3] X. Shi, S. Tan, & S. Tan, Nlrp3 inflammasome in sepsis (review), Molecular Medicine Reports, vol. 24, no. 1, 2021. https://doi.org/10.3892/mmr.2021.12153
[4] N. Kelley, D. Jeltema, Y. Duan, & Y. He, The nlrp3 inflammasome: an overview of mechanisms of activation and regulation, International Journal of Molecular Sciences, vol. 20, no. 13, p. 3328, 2019. https://doi.org/10.3390/ijms20133328
[5] L. El-Sharkawy, D. Brough, & S. Freeman, Inhibiting the nlrp3 inflammasome, Molecules, vol. 25, no. 23, p. 5533, 2020. https://doi.org/10.3390/molecules25235533
[6] N. Song and T. Li, Regulation of nlrp3 inflammasome by phosphorylation, Frontiers in Immunology, vol. 9, 2018. https://doi.org/10.3389/fimmu.2018.02305
[7] A. Akbal, A. Dernst, M. Lovotti, M. Mangan, R. McManus, & E. Latz, How location and cellular signaling combine to activate the nlrp3 inflammasome, Cellular and Molecular Immunology, vol. 19, no. 11, p. 1201-1214, 2022. https://doi.org/10.1038/s41423-022-00922-w
[8] Z. Zhong, S. Liang, E. Sánchez-López, F. He, S. Shalapour, X. Linet al., New mitochondrial dna synthesis enables nlrp3 inflammasome activation, Nature, vol. 560, no. 7717, p. 198-203, 2018. https://doi.org/10.1038/s41586-018-0372-z