The TIGIT target, once hailed as the "next PD-1", has garnered significant attention in the field of immunotherapy. However, its development journey has been fraught with challenges, with numerous pharmaceutical companies encountering setbacks. Recently, AstraZeneca initiated a Phase III head-to-head study of its PD-1/TIGIT bispecific antibody, rilvegostomig, against pembrolizumab (Keytruda), bringing renewed hope to the TIGIT target. Whether TIGIT can break the “undruggable” spell is a question that not only concerns the future of the TIGIT target itself but also impacts the landscape of the entire immunotherapy field.
1. The Introduction of TIGIT
TIGIT (T cell immunoreceptor with Ig and ITIM domains) is a significant immune checkpoint molecule that features an immunoglobulin-like domain and an immunoreceptor tyrosine-based inhibitory motif (ITIM), belonging to the immunoglobulin superfamily. TIGIT is expressed on NK cells, T cells, and dendritic cells (DCs). Its primary function is to transmit inhibitory signals by interacting with its ligands CD155 and CD112, which suppress the activation and effector functions of T cells and NK cells, reducing inflammatory responses and immune reactions, and aiding tumor cells in evading immune surveillance. By blocking the binding of TIGIT to its ligands, the antitumor activity of T cells and NK cells can be restored or enhanced, thereby improving the efficacy of immunotherapy. Consequently, TIGIT has emerged as a novel target in cancer immunotherapy [1].
2. TIGIT Works as an Immune Checkpoint Receptor
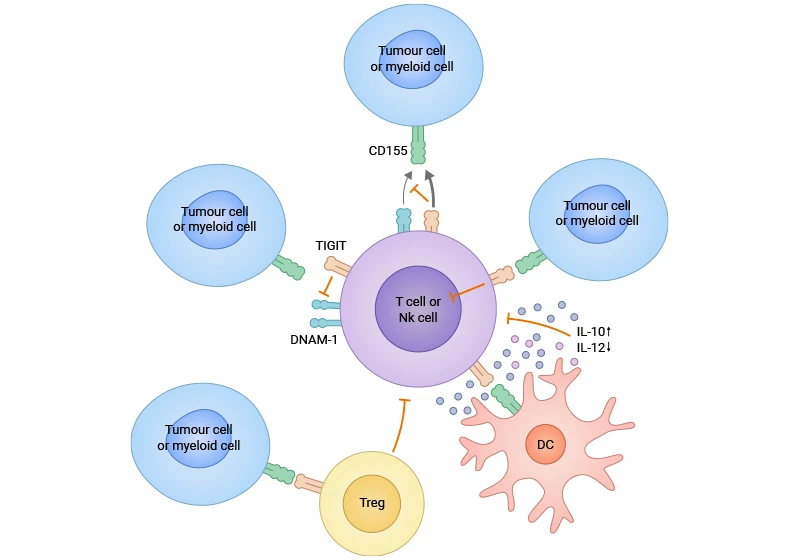
TIGIT is a key receptor found on activated T cells and NK cells. It binds to proteins on tumor cells, which sends signals to weaken these immune cells and helps tumors avoid being attacked. TIGIT affects the immune response in several ways: it curbs NK cell cytotoxicity, limiting tumor cell death and antigen presentation; it also impairs dendritic cell function, reducing T cell priming; and it directly suppresses the activity of CD8+ T cells. TIGIT-expressing regulatory T cells (Tregs) amplify this suppression. Targeting TIGIT with antibodies is a novel approach in cancer immunotherapy, as high TIGIT levels correlate with poorer patient outcomes. By preventing TIGIT from binding its ligands, the activity of NK cells and CD8+ T cells can be enhanced, improving the antitumor immune response (Figure 1) [2-4].
Figure 1. TIGIT exerts its effects through multiple mechanisms in the cancer [7]
3. TIGIT is Linked to Cancer, Autoimmune Diseases, and Chronic Viral Infections
3.1 Cancer
In non-small cell lung cancer (NSCLC), TIGIT is closely related to tumor prognosis, which has been confirmed in multiple studies. TIGIT, an immune checkpoint molecule, is crucial in modulating both adaptive and innate immune responses. Studies have shown that TIGIT levels in tumor-infiltrating lymphocytes (TILs) of NSCLC patients are markedly higher than in healthy individuals, and its mRNA levels increase more rapidly than those of PD-1, suggesting a potentially more significant role for TIGIT in NSCLC. High TIGIT expression is also related to other cancers, including renal cell carcinoma, melanoma, esophageal cancer, colorectal cancer, breast cancer, glioblastoma, gastric cancer, gastroesophageal junction cancer, and head and neck squamous cell carcinoma. These observations highlight the growing interest in TIGIT's expression patterns and clinical implications across various cancers, positioning it as a potential key target for future cancer therapeutics [5-9].
3.2 Autoimmune Diseases
In autoimmune diseases, like Systemic Lupus Erythematosus (SLE) and Autoimmune Hepatitis (AIH), research indicates that elevated TIGIT expression is associated with reduced disease activity, implying that TIGIT might help suppress the activity of self-reactive T cells and preserve immune tolerance. Therapies targeting TIGIT are under investigation, with the potential to restore Treg function and alleviate autoimmune symptoms. For example, in a mouse model of lupus, the use of TIGIT-Ig fusion protein has been shown to delay proteinuria onset, significantly lower autoantibody levels, and enhance survival rates. These findings suggest that TIGIT targeting could enhance self-antigen tolerance and provide a novel approach to treating autoimmune diseases [10-11].
3.3 Chronic Viral Infections
Chronic Hepatitis B Virus (HBV) infection, a global health concern, is intricately linked to the host's immune system dynamics. Evidence suggests that ongoing HBV infection modifies the expression of immune cell receptors, triggering immune dysregulation that facilitates viral evasion and sustained infection. Notably, the increased expression of TIGIT is identified as a key contributor to immune dysfunction in these patients. TIGIT, highly expressed in natural killer (NK) cells and T lymphocytes, suppresses their activity, diminishing the ability to clear HBV. Consequently, targeting TIGIT could potentially restore immune function and enhance antiviral responses in affected individuals [12-13].
4. Advances in TIGIT Drug Research
As a target for immunotherapy, TIGIT has more than 100 clinical studies underway, with drug types including monoclonal antibodies, bispecific antibodies, small molecules, and fusion proteins, primarily applied in cancer disease treatment. Among them, nearly 40 monoclonal antibodies targeting TIGIT and over 10 bispecific antibodies containing the TIGIT target have entered Phase III or Phase II clinical trials (Table 1). These drugs primarily function by blocking the signaling pathways of TIGIT and its ligands such as CD155 and CD112, which promotes the activation and proliferation of T cells and NK cells, thereby enhancing the immune system's ability to attack cancer.
The focus on various new drugs targeting TIGIT alone or in combination with PD-1/PD-L1, is gaining the attention of international pharmaceutical companies like Roche, Merck, and BMS. These clinical trials are categorized into three types: 1) Concurrent administration of anti-TIGIT and anti-PD-1/PD-L1 drugs (e.g., Tiragolumab plus Atezolizumab); 2) Combination formulations of anti-TIGIT and anti-PD-1/PD-L1 drugs (e.g., the DZ combination of Domvanalimab and Zimberelimab; the combination of MK-7684 A, pembrolizumab, and vibostolimab); 3) Bispecific antibodies combining TIGIT and PD-1/PD-L1 (such as AZD 2936, IBI321). Ongoing global research into the role of TIGIT in immune response regulation will expand to more cancer types and other diseases, ultimately offering more effective treatments to patients worldwide.
|
Drug
|
target
|
Drug type
|
Indications
|
Institutions
|
Highest clinical phase
|
|
Belrestotug
|
TIGIT
|
monoclonal antibody
|
PD-L1 positive non-small cell lung cancer | PD-L1 positive esophageal squamous cell carcinoma | head and neck squamous cell carcinoma
|
GSK Plc | iTeos Belgium SA | iTeos Therapeutics, Inc.
|
Clinical stage 3
|
|
Domvanalimab
|
TIGIT
|
monoclonal antibody
|
Esophageal adenocarcinoma | progressive gastric adenocarcinoma
|
Arcus Biosciences, Inc. | Gilead Sciences, Inc.
|
Clinical stage 3
|
|
Ospitalian monoclonal antibody
|
TIGIT
|
monoclonal antibody
|
Non small cell lung cancer | tumor | advanced esophageal squamous cell carcinoma | hepatocellular carcinoma
|
Baiji Shenzhou (Guangzhou) Innovation and Technology Co., Ltd.
|
Clinical stage 3
|
|
Pembrolizumab/vibostolimab
|
PD-1 x TIGIT
|
monoclonal antibody
|
Melanoma | Non-small cell lung cancer | Extensive small cell lung cancer | Ovarian cancer | Breast cancer | Cervical cancer | Advanced urothelial cancer
|
Merck Sharp & Dohme Corp.
|
Clinical stage 3
|
|
Rilvegostomig
|
PD-1 x TIGIT
|
Bispecific antibody
|
Metastatic non-squamous non-small cell lung cancer | Advanced biliary carcinoma | Biliary carcinoma | Gastroesophageal junction adenocarcinoma | Renal tumor | Bladder cancer
|
AstraZeneca PLC | AstraZeneca AB
|
Clinical stage 3
|
|
Tiragolumab
|
TIGIT
|
monoclonal antibody
|
PD-L1 positive non-small cell lung cancer | hepatocellular carcinoma | esophageal squamous cell carcinoma
|
Hoffmann-La Roche, Inc.
|
Clinical stage 3
|
|
Etigilimab
|
TIGIT
|
monoclonal antibody
|
Clear cell adenocarcinoma of fallopian tube | recurrent clear cell adenocarcinoma of ovary | recurrent platinum-resistant fallopian tube carcinoma | recurrent platinum-resistant ovarian carcinoma
|
The University of Texas MD Anderson Cancer Center | Mereo BioPharma Group Plc
|
Clinical stage 2
|
|
HLX-301
|
PDL1 x TIGIT
|
Bispecific antibody
|
Solid Tumor | Lymphoma | Tumor Metastasis | Non-small Cell Lung Cancer
|
Shanghai Fosun Pharmaceutical Industry Development Co., Ltd. | Shanghai Fuhong Hanlin Biotechnology Co., Ltd.
|
Clinical stage 2
|
|
Recombinant humanized anti-TIGIT monoclonal antibody (shanghai junshi organism)
|
TIGIT
|
monoclonal antibody
|
Solid tumor | small cell lung cancer
|
Shanghai Junshi Biomedical Technology Co., Ltd. | Coherus BioSciences, Inc
|
Clinical stage 2
|
|
Renvistobart
|
TIGIT
|
monoclonal antibody
|
Metastatic non-small cell lung cancer | solid tumor | endometrial carcinoma | head and neck tumor | ovarian cancer | multiple myeloma | advanced malignant solid tumor
|
Bristol Myers Squibb Co. | Compugen Ltd. | Sino-US Shanghai Squibb Pharmaceutical Co., Ltd.
|
Clinical stage 2
|
|
ZG-005
|
PD-1 x TIGIT
|
Bispecific antibody
|
Advanced hepatocellular carcinoma | Advanced neuroendocrine carcinoma | Advanced cervical carcinoma | Advanced malignant solid tumor | Solid tumor
|
Suzhou zejing biopharmaceutical co., ltd
|
Clinical stage 2
|
|
HB-0036
|
PDL1 x TIGIT
|
Bispecific antibody
|
Advanced malignant solid tumor | non-small cell lung cancer | solid tumor
|
Shanghai huaaotai biological pharmaceutical co., ltd
|
Clinical stage 1/2
|
|
Lancastotug
|
TIGIT
|
monoclonal antibody
|
Advanced malignant solid tumor
|
Zhongshan kangfang biological medicine co., ltd
|
Clinical stage 1/2
|
|
PM-1009
|
CD112R x TIGIT
|
Bispecific antibody
|
Metastatic hepatocellular carcinoma | hepatocellular carcinoma | advanced cancer | locally advanced hepatocellular carcinoma | lung cancer
|
Pumice Biotechnology (Zhuhai) Co., Ltd.
|
Clinical stage 1/2
|
|
PM-1022
|
PDL1 x TIGIT
|
Bispecific antibody
|
Advanced cancer
|
Pumice Biotechnology (Zhuhai) Co., Ltd. | Adimab LLC
|
Clinical stage 1/2
|
|
Vibostolimab
|
TIGIT
|
monoclonal antibody
|
Blood tumor
|
Merck Sharp & Dohme Corp.
|
Clinical stage 1/2
|
|
AB-308
|
TIGIT
|
monoclonal antibody
|
Advanced malignant solid tumor | diffuse large B cell lymphoma | esophageal cancer | adenocarcinoma at the junction of gastroesophageal junction | melanoma | multiple myeloma
|
Arcus Biosciences, Inc.
|
Clinical stage 1
|
|
ASP-8374
|
TIGIT
|
monoclonal antibody
|
Recurrent glioblastoma
|
Regeneron Pharmaceuticals, Inc. | Astellas Pharma, Inc.
|
Clinical stage 1
|
|
BAT-6005
|
TIGIT
|
monoclonal antibody
|
Advanced malignant solid tumor
|
Baiaotai biopharmaceuticals co., ltd
|
Clinical stage 1
|
|
BC008-1A
|
PD-1 x TIGIT
|
Bispecific antibody
|
Advanced malignant solid tumor
|
Shandong Buchang Pharmaceutical Co., Ltd. | Sichuan Luzhou Buchang Biological Pharmaceutical Co., Ltd.
|
Clinical stage 1
|
Table 1. Part TIGIT Clinical Drugs in Progress
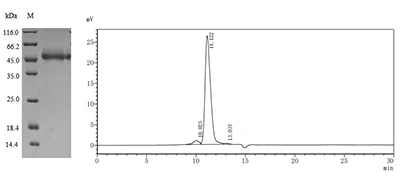
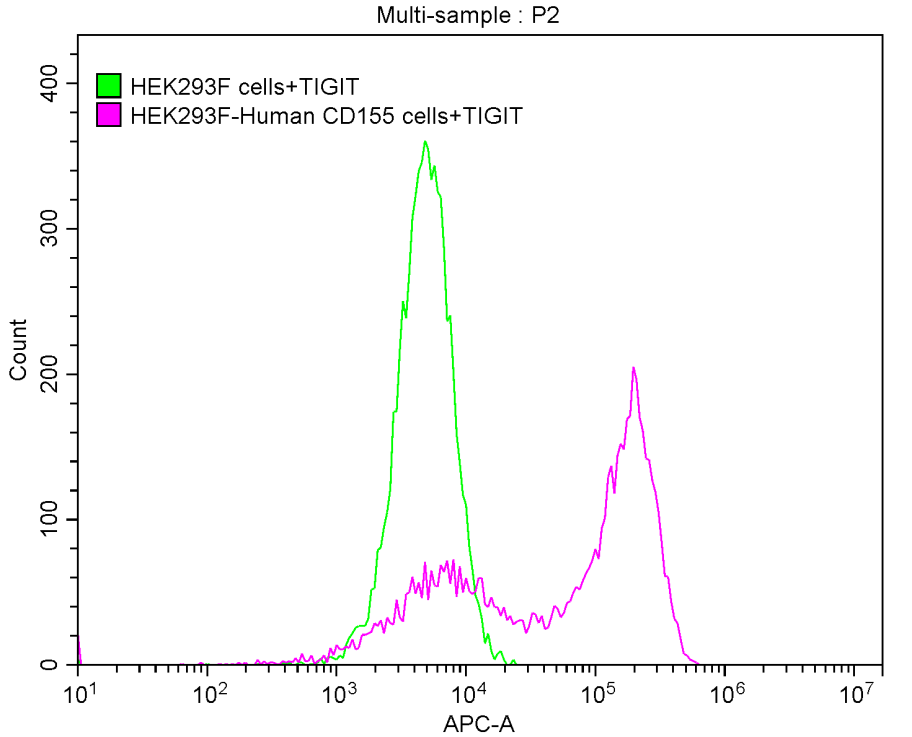
5. CUSABIO TIGIT Recombinant Protein for Research Use
To assist pharmaceutical companies in their clinical research on TIGIT in cancer, autoimmune diseases, chronic viral infections, and especially in combined applications of TIGIT with PD-1/PD-L1 inhibitors, CUSABIO has launched high-activity TIGIT protein products. The offerings aim to facilitate your exploration of the mechanisms and potential clinical value of TIGIT.
References
[1] Zhang, X., et al. "TIGIT: An emerging immune checkpoint target for immunotherapy in cancer." Frontiers in Immunology 14 (2023): 123-135.
[2] Harjunp, H., and C. Guillerey. "TIGIT as an emerging immune checkpoint." Clinical & Experimental Immunology 200.2 (2020): 108-119.
[3] Chauvin, Joe-Marc, and Hassane M. Zarour. "TIGIT in cancer immunotherapy." Journal for immunotherapy of cancer 8.2 (2020).
[4] Chu, Xianjing, et al. "Co-inhibition of TIGIT and PD-1/PD-L1 in cancer immunotherapy: mechanisms and clinical trials." Molecular cancer 22.1 (2023): 93.
[5] Sun, Yu, et al. "Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma)." Cancer Letters 471 (2020): 1-10.
[6] Lee, Jiyoung, et al. "Prognostic impact of PD-L1 and TIGIT expression in non-small cell lung cancer." Scientific Reports 13 (2023): 29724.
[7] Xiao, Kunmin, et al. "Prognostic Role of TIGIT Expression in Patients with Solid Tumors: A Meta-Analysis." BioMed Research International 2021 (2021): 5440572.
[8] Kong, Yaxian, et al. "T-cell immunoglobulin and ITIM domain (TIGIT) associates with CD8+ T-cell exhaustion and poor clinical outcome in AML patients." Clinical Cancer Research 22.12 (2016): 3057-3066.
[9] Wen, Jie, et al. "A pan-cancer analysis revealing the role of TIGIT in tumor microenvironment." Scientific Reports 11.1 (2021): 22502.
[10] Yue, Y., et al. "TIGIT as a Promising Therapeutic Target in Autoimmune Diseases." Frontiers in Immunology 12 (2021): 699895.
[11] Kojima, Marenori, et al. "Anti-human-TIGIT agonistic antibody ameliorates autoimmune diseases by inhibiting Tfh and Tph cells and enhancing Treg cells." Communications biology 6.1 (2023): 500.
[12] Wei, Yan-Yan, et al. "TIGIT marks exhausted T cells and serves as a target for immune restoration in patients with chronic HBV infection." American Journal of Translational Research 14.2 (2022): 942.
[13] Yu, Lihua, et al. "TIGIT+ TIM-3+ NK cells are correlated with NK cell exhaustion and disease progression in patients with hepatitis B virus‑related hepatocellular carcinoma." Oncoimmunology 10.1 (2021): 1942673.
CUSABIO team. TIGIT Targeted Immunotherapies Offer New Hope for Cancer Treatment. https://www.cusabio.com/c-21202.html



Comments
Leave a Comment