1. What is SEZ6?
Seizure-related 6 homolog (SEZ6) is a transmembrane protein initially discovered due to its association with epilepsy [1]. The gene is located on chromosome 17q21.33 and encodes a protein with multiple domains that plays important roles in the nervous system.
The expression of SEZ6 is tissue-specific. In normal tissues, SEZ6 is mainly expressed in the brain (such as the cerebellum, cerebral cortex, and spinal cord) and the pituitary gland, while its expression is extremely low in other tissues (such as the lung, liver, and kidney) [2,3]. RNA sequencing and immunohistochemical (IHC) analyses have shown that SEZ6 is highly expressed in neuroendocrine tumors such as small cell lung cancer (SCLC) and neuroendocrine carcinomas (NECs), and is highly correlated with the expression of neuroendocrine markers such as chromogranin A (CHGA) [2]. In 73 primary SCLC samples, approximately 78% of the tumors exhibited SEZ6-positive expression, with an average positivity rate of 40.4%, indicating a high prevalence of SEZ6 in SCLC [2].
Additionally, SEZ6 is expressed in the neurosensory hair cells of the mouse inner ear, and its expression persists from the embryonic, postnatal to the adult stages, suggesting its potential role in the development and functional maintenance of the auditory system [3].
2. Mechanisms of SEZ6 Research
SEZ6 is an important substrate of β-secretase (BACE1). BACE1 can cleave the extracellular domain of SEZ6, producing a soluble ectodomain (sSEZ6) and a transmembrane C-terminal fragment (SEZ6-CTF); subsequently, SEZ6-CTF can be further cleaved by γ-secretase to release the intracellular domain (SEZ6-ICD) [4]. This cleavage process occurs highly specifically in neurons and is crucial for the function of SEZ6.
In SCLC, SEZ6, as a cell surface protein, can be specifically recognized and rapidly internalized by antibodies (such as SC17), making it an ideal target for antibody-drug conjugates (ADCs) [2]. Studies have shown that ADCs targeting SEZ6 (such as ABBV-011) can internalize into tumor cells, releasing cytotoxic payloads (such as calicheamicin) to specifically kill SEZ6-positive tumor cells [2].
3. SEZ6-Related Signaling Pathways
3.1 BACE1-SEZ6-γ-secretase Pathway
This pathway is the core signaling pathway of SEZ6. The SEZ6-CTF generated by BACE1 cleavage of SEZ6 can transmit signals by binding to ERM proteins, regulating cytoskeleton reorganization (such as growth cone collapse); further cleavage of SEZ6-CTF by γ-secretase can terminate this signal, allowing the cell to regain its responsiveness to new signals [4]. In neurons, this pathway is involved in axon guidance and synaptic plasticity regulation [4].
3.2 Association with Neuroendocrine Signaling
In SCLC, the expression of SEZ6 is highly correlated with neuroendocrine markers such as CHGA [2], suggesting its potential involvement in the signaling pathways regulated by ASCL1 (a transcription factor that drives the fate of neuroendocrine cells). Studies have shown that SEZ6 is a downstream target gene of ASCL1 and is significantly upregulated in SCLC subtypes with high ASCL1 expression [2].
3.3 Auditory Signaling Pathway
In the mouse inner ear, SEZ6 is expressed in the stereocilia and cytoplasm of hair cells, and may participate in the transmission and integration of auditory signals by regulating ion channels (such as potassium channels) [3]. Mutations in SEZ6 may affect the structure and function of hair cells, leading to hearing impairments.
4. SEZ6-Related Diseases
4.1 Tumors
4.1.1 Small Cell Lung Cancer (SCLC)
SEZ6 is highly expressed in SCLC and is associated with poor prognosis — patients with high SEZ6 expression in SCLC have shorter survival periods [2]. Mechanistically, SEZ6 may interact with ezrin-radixin-moesin (ERM) family proteins to affect the cytoskeleton dynamics of tumor cells, thereby promoting tumor growth and invasion [2,3]. Based on its high specificity and internalization characteristics, ADCs targeting SEZ6 (such as ABBV-011, ABBV-706) have entered clinical trials for the treatment of relapsed/refractory SCLC [2,6].
4.1.2 Neuroendocrine Carcinomas (NECs)
SEZ6 is also highly expressed in NECs and is a potential therapeutic target, with related combination therapies (such as with PD-1 inhibitors, platinum drugs) under investigation [6].
4.2 Hearing Impairment
A homozygous missense mutation in SEZ6 (such as c.2092G>A:p.Val698Ile) is associated with autosomal recessive nonsyndromic hearing impairment. In a consanguineous Pakistani family, this mutation leads to abnormal SEZ6 protein structure, affecting the function of inner ear hair cells and resulting in congenital severe sensorineural deafness [3]. SEZ6 is expressed in the neurosensory hair cells of the mouse inner ear and persists from the embryonic, postnatal to the adult stages, indicating its role in the development and functional maintenance of the auditory system [3].
4.3 Neurological Diseases
4.3.1 Epilepsy
SEZ6 was initially named due to its association with epilepsy, and its abnormal expression may participate in epileptic seizures by affecting neuronal excitability [1,7].
4.3.2 Alzheimer’s Disease (AD)
SEZ6 is a substrate of BACE1, which is a key enzyme in the production of Aβ in AD. BACE1 inhibitors can reduce the cleavage of SEZ6, leading to impaired synaptic plasticity (such as reduced LTP), suggesting that SEZ6 may be involved in synaptic dysfunction in AD [4,8]. SEZ6 knockout mice exhibit reduced dendritic branching of cortical pyramidal cells, abnormal excitability, decreased dendritic spine density in the hippocampal region, and spatial memory deficits [4].
4.3.3 Cognitive and Behavioral Abnormalities
SEZ6 knockout mice display cognitive deficits, motor coordination disorders, and abnormal emotional responses, which are related to the phenotypes of intellectual disability and schizophrenia in humans [4]. SEZ6 regulates the glycosylation and trafficking of kainate receptor subunits GluK2 and GluK3, affecting synaptic transmission, which may be one of the mechanisms underlying its cognitive abnormalities [3].
5. Research Progress on SEZ6 Targeted Drugs
SEZ6 is highly expressed in neuroendocrine tumors (such as SCLC) but lowly expressed in normal tissues, making it a popular target for antibody-drug conjugates (ADCs). Several ADC pipelines are currently under development, mainly for the treatment of small cell lung cancer, with the highest development stage being Phase 1 clinical trials. Some of the ongoing pipelines are listed below:
| drugs |
Mechanism of action |
Type of medication |
Indications under investigation (disease name) |
Institutions under research |
Highest R&D stage |
| SC-011 |
DNA inhibitors | SEZ6 inhibitors |
ADC |
Refractory small cell lung cancer |
AbbVie, Inc. |
Phase 1 clinical trial |
| ABBV-706 |
SEZ6 inhibitor | TOP1 inhibitors |
ADC |
Advanced malignant solid tumors | Small cell lung cancer | Recurrent small cell lung cancer | Refractory small cell lung cancer | Non-small cell lung cancer |
AbbVie, Inc. |
Phase 1 clinical trial |
| DLL3xSEZ6 bsADC(Biocytogen) |
DLL3 inhibitor | SEZ6 inhibitor | TOP1 inhibitors |
ADC |
Small cell lung cancer |
Biocytogen Boston Corp. |
Preclinical |
| DEC-002 |
SEZ6 inhibitors |
ADC |
tumor |
Wuxi Biologics Co., Ltd. | Whitehawk Therapeutics, Inc. | Hangzhou Duoxi Biotechnology Co., Ltd |
Preclinical |
| HWK-206 |
SEZ6 modulator |
ADC |
Neuroendocrine carcinoma | Small cell lung cancer |
Whitehawk Therapeutics, Inc. |
Preclinical |
(Data source: Patsnap)
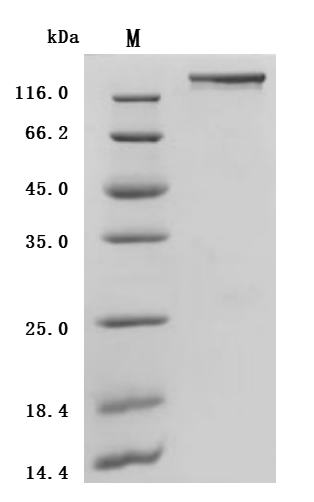
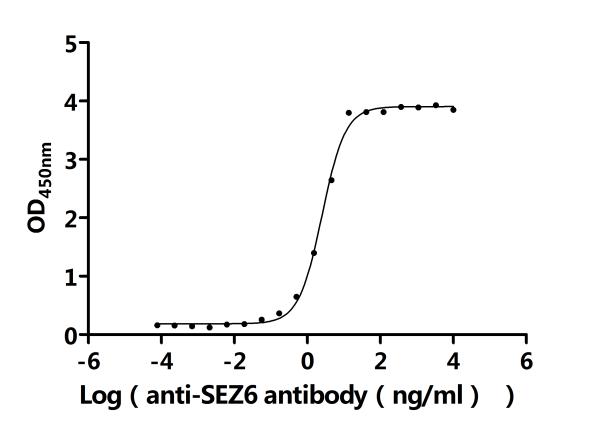
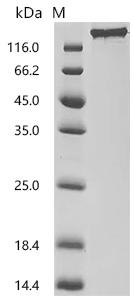
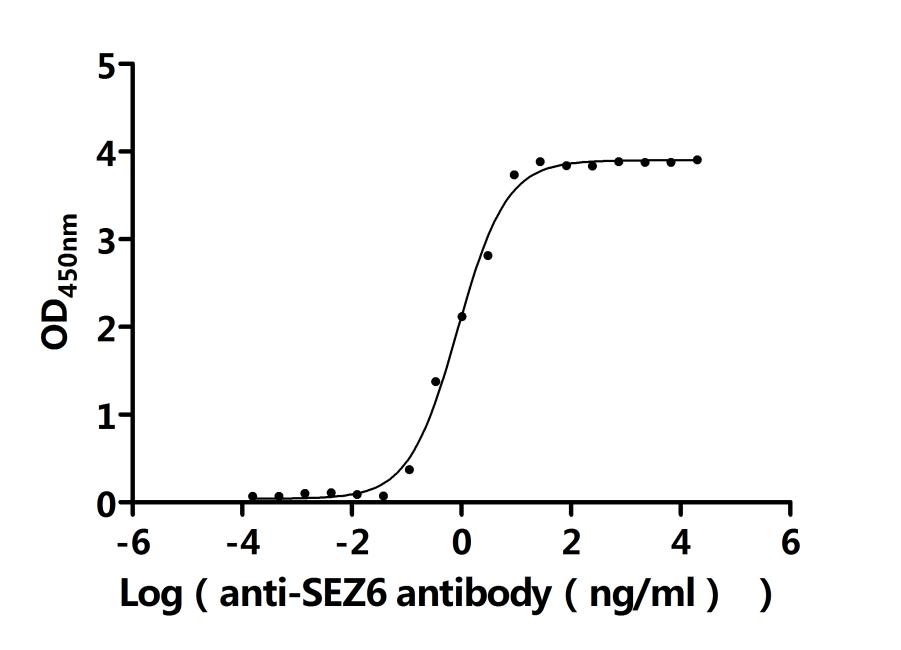
6. CUSABIO SEZ6 Research-Related Product Recommendations
References
[1] Gunnersen JM, Kim MH, Fuller SJ, et al. Sez-6 proteins affect dendritic arborization patterns and excitability of cortical pyramidal neurons. Neuron, 2007, 56: 621-639.
[2] Wiedemeyer WR, Gavrilyuk J, Schammel A, et al. ABBV-011, A Novel, Calicheamicin-Based Antibody–Drug Conjugate, Targets SEZ6 to Eradicate Small Cell Lung Cancer Tumors. Molecular Cancer Therapeutics, 2022, 21(6): 986-998.
[3] Bharadwaj T, Schrauwen I, Rehman S, et al. ADAMTS1, MPDZ, MVD, and SEZ6: candidate genes for autosomal recessive nonsyndromic hearing impairment. European Journal of Human Genetics, 2022, 30: 22-33.
[4] Shimizu-Nishikawa K, Kajiwara K, Kimura M, et al. Cloning and expression of SEZ-6, a brain-specific and seizure-related cDNA. Brain Res Mol Brain Res, 1995, 28: 201-210.
[5] Ohno M, Sametsky EA, Younkin LH, et al. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer's disease. Neuron, 2004, 41: 27-33.
[6] Chandana S, Garmezy B, Dowlati A, et al. Phase I study of ABBV-706, an anti-SEZ6 antibody-drug conjugate, alone or in combination in adults with advanced solid tumors. Annals of Oncology, 2023, 34(S2): S1077.
[7] Pigoni M, Hsia HE, Hartmann J, et al. Seizure protein 6 controls glycosylation and trafficking of kainate receptor subunits GluK2 and GluK3. EMBO J, 2020, 39: e103457.
[8] Zhu K, Xiang X, Filser S, et al. BACE1 Inhibition Impairs Synaptic Plasticity via Seizure Protein 6. Biological Psychiatry, 2017, doi:10.1016/j.biopsych.2016.12.023.
CUSABIO team. SEZ6: A New Therapeutic Target for Neuroendocrine Tumors with Multiple ADCs in Clinical Development. https://www.cusabio.com/c-21255.html

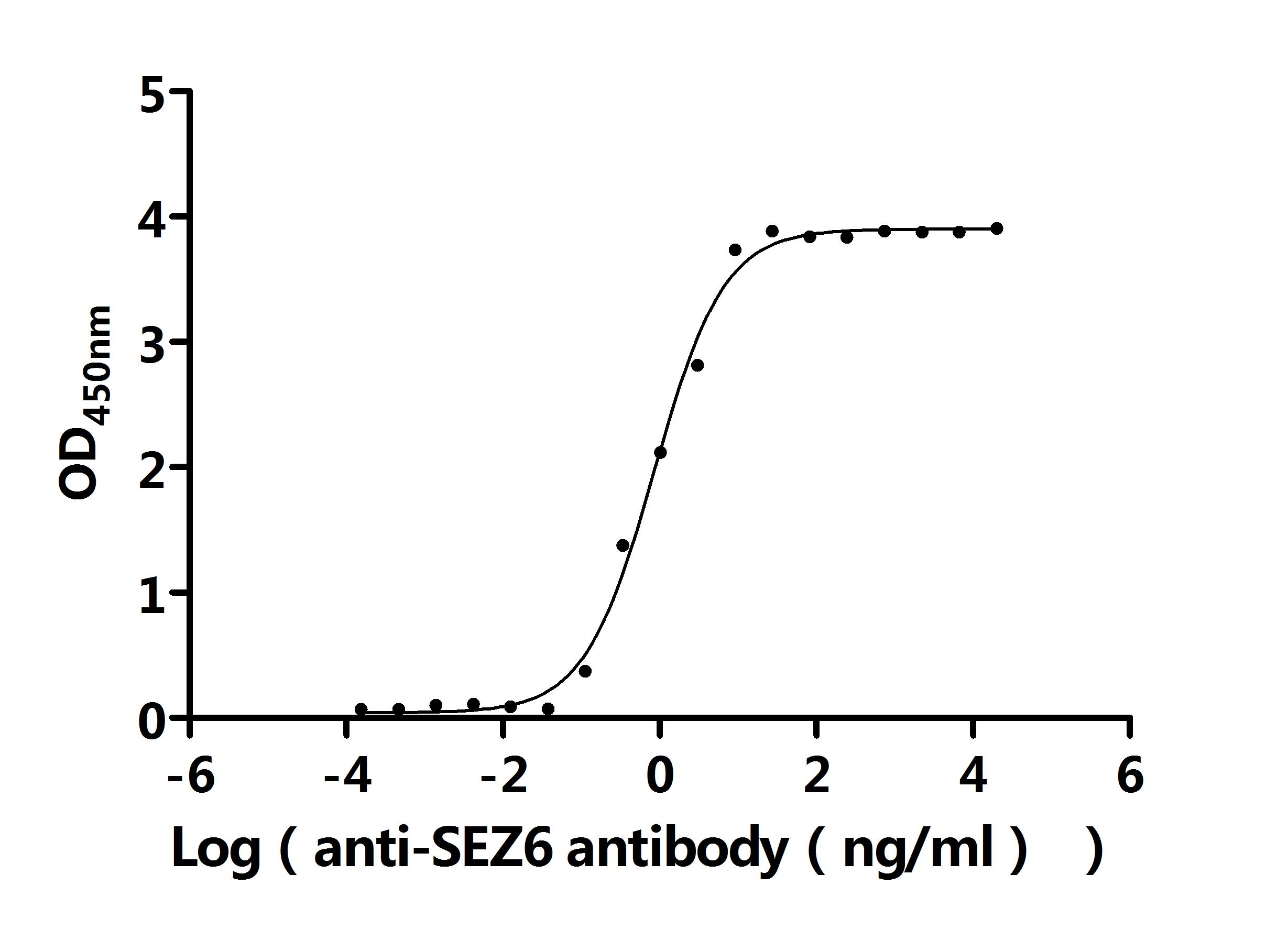
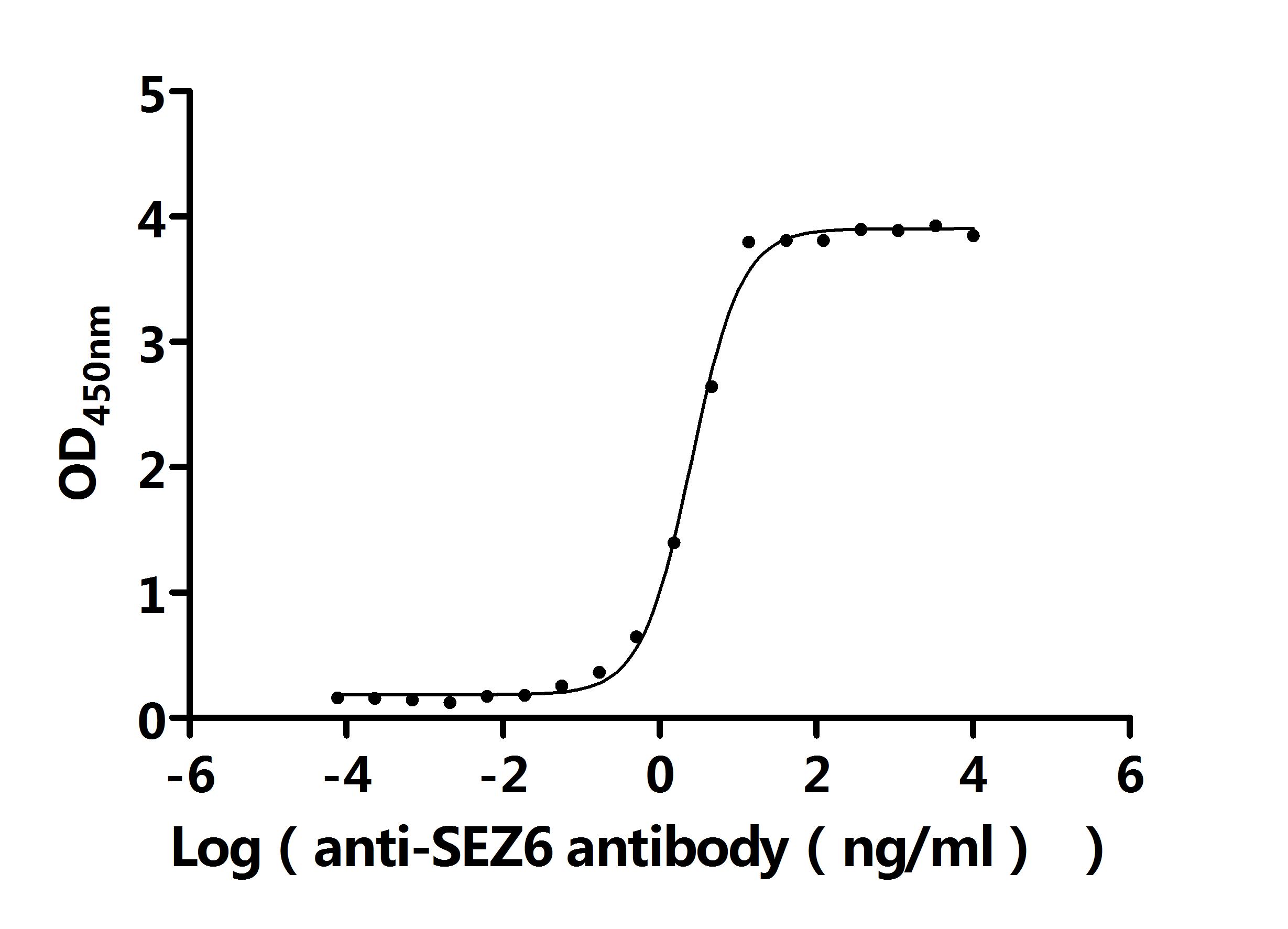
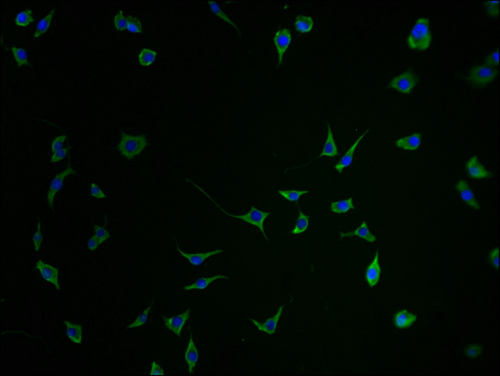



Comments
Leave a Comment