[1] Rasmus S Pedersen, M. Karsdal, N. Willumsen.(2024). Abstract 4287: Serological quantification of fibroblast activation protein (FAP) cleaved type III collagen: A biomarker for FAP activity.
[2] Sebastian Dziadek, A. Kraxner, Wei-Yi Cheng, Tai-Hsien Ou Yang, Mike Flores, Noah Theiss, T. Tsao, Emilia Andersson, S. V. Harring, Ann-Marie E. Bröske, Maurizio Ceppi, Volker Teichgräber, Jehad Charo.(2024). Comprehensive analysis of fibroblast activation protein expression across 23 tumor indications: insights for biomarker development in cancer immunotherapies.
[3] K. Hartmann, Merel van Gogh, P. C. Freitag, Florian Kast, Gabriela Nagy-Davidescu, L. Borsig, A. Plückthun.(2022). FAP-retargeted Ad5 enables in vivo gene delivery to stromal cells in the tumor microenvironment.
[4] Zihan Wang, Jinping Wang, Tianyi Lan, Liubo Zhang, Zeran Yan, N. Zhang, Yuan Xu, Qing-wen Tao.(2023). Role and mechanism of fibroblast-activated protein-α expression on the surface of fibroblast-like synoviocytes in rheumatoid arthritis.
[5] Spencer D. Lindeman, Jack Higgins.(2024). Abstract 6026: A novel lutetium-177 radioligand therapy targeting FAP has potent antitumor activity in xenograft cancer model.
[6] Ruchi Shah, Katherine A. Johnson, Anna E L Lippert, Sean G Kraus, Philip B. Emmerich, Cheri A Pasch, Wei Zhang, K. Matkowskyj, Aaron M LeBeau, D. Deming.(2024). Cancer-Associated Fibroblast Proteins as Potential Targets against Colorectal Cancers.
[7] Mengxin Xu, Pu Zhang, J. Ding, Junyi Chen, L. Huo, Zhibo Liu.(2021). Albumin Binder–Conjugated Fibroblast Activation Protein Inhibitor Radiopharmaceuticals for Cancer Therapy.
[8] Layne N. Raborn, Z. Michel, Michael T. Collins, Alison M Boyce, L. F. de Castro.(2024). Fibroblast Activation Protein Is Expressed by Altered Osteoprogenitors and Associated to Disease Burden in Fibrous Dysplasia.
[9] L. Loureiro, Lydia Hoffmann, Christin Neuber, Luise Rupp, C. Arndt, A. Kegler, M. Kubeil, Christoph E Hagemeyer, Holger Stephan, Marc Schmitz, A. Feldmann, M. Bachmann.(2023). Immunotheranostic target modules for imaging and navigation of UniCAR T-cells to strike FAP-expressing cells and the tumor microenvironment.
[10] Yentl Van Rymenant, Anke de Groot, Laura Dirkx, Emile Verhulst, Joni De Loose, Isabel Pintelon, Tias Verhezen, J. De Waele, Sofie Thys, O. De Wever, Muhammet Tanc, G. Caljon, Pieter Van der Veken, Ingrid De Meester.(2025). FAP on human NK cells: insights from NK cell activation and crosstalk with cancer-associated fibroblasts.
[11] Lirong Gao, Anqi Wang, Yuling Chen, Xin Cai, Yue Li, Jian Zhao, Yang Zhang, Weijie Zhang, Jianjie Zhu, Yuanyuan Zeng, Zeyi Liu, Jianyang Huang.(2023). FTO facilitates cancer metastasis by modifying the m6A level of FAP to induce integrin/FAK signaling in non-small cell lung cancer.
[12] Patrizio Ansalone.(2014). Electrostatic Affinities and Binding Kinetics among α2I Integrin Domains, Divalent Cations and 21-mer Collagen Fragment.
[13] Samuel Bell, Eugene M. Terentjev.(2016). Focal adhesion kinase - the reversible molecular mechanosensor.
[14] Min Li, Xue Cheng, Rong Rong, Yan-ping Gao, Xiuwu Tang, Youguo Chen.(2020). High expression of fibroblast activation protein (FAP) predicts poor outcome in high-grade serous ovarian cancer.
[15] Monika Licaj, R. Mhaidly, Y. Kieffer, H. Croizer, C. Bonneau, A. Meng, L. Djerroudi, Kevin Mujangi-Ebeka, H. Hocine, B. Bourachot, Ilaria Magagna, Renaud Leclère, Léa Guyonnet, Mylène Bohec, Coralie Guérin, S. Baulande, M. Kamal, C. le Tourneau, Fabrice Lécuru, Véronique Becette, Roman Rouzier, A. Vincent-Salomon, Géraldine Gentric, F. Mechta-Grigoriou.(2024). Residual ANTXR1+ myofibroblasts after chemotherapy inhibit anti-tumor immunity via YAP1 signaling pathway.
[16] Q. Ping, Chunhui Wang, Xin Cheng, Yiming Zhong, R. Yan, Meng Yang, Yunqiang Shi, Xiangmeng Li, Xiao Li, Wenwen Huang, Liqiong Wang, Xiaofang Bi, Libing Hu, Yang Yang, Yingbao Wang, R. Gong, Jun Tan, Rui Li, Hui Li, Jian Li, Wenju Wang, Ruhong Li.(2023). TGF-β1 dominates stromal fibroblast-mediated EMT via the FAP/VCAN axis in bladder cancer cells.
[17] Linlin Wang, Dong Yang, Jing Tian, Aiqin Gao, Yihang Shen, X. Ren, Xia Li, G. Jiang, Taotao Dong.(2017). Tumor necrosis factor receptor 2/AKT and ERK signaling pathways contribute to the switch from fibroblasts to CAFs by progranulin in microenvironment of colorectal cancer.
[18] Ruifang Li, Yifan Tai, Xinyan Zhang, Zhen Liu, Haipeng Si, Deling Kong, Lili Zhao, Jia Li, Adam C. Midgley.(2025). Tissue‐Microenvironment‐Responsive Self‐Assembling Peptide Nanoshells Boost Pirfenidone Efficacy in the Treatment of Liver Fibrosis.
[19] Jiawan Wang, Heng Du, Wanrun Xie, Jinmiao Bi, Hao Zhang, Xu Liu, Yuhan Wang, Shaolong Zhang, Anhua Lei, Chuting He, Hailong Yuan, Jiahe Zhang, Yujing Li, Pengfei Xu, Siqi Liu, Yanan Zhou, Jianghua Shen, Jingdong Wu, Yihong Cai, Chaofan Yang, Zeya Li, Y. Liang, Yang Zhao, Jin Zhang, Moshi Song.(2024). CAR-Macrophage Therapy Alleviates Myocardial Ischemia-Reperfusion Injury.
[20] Shipra Das, J. Valton, P. Duchateau, L. Poirot.(2023). Stromal depletion by TALEN-edited universal hypoimmunogenic FAP-CAR T cells enables infiltration and anti-tumor cytotoxicity of tumor antigen-targeted CAR-T immunotherapy.
[21] Tingting You, Huijing Tang, Wenjing Wu, Jin Gao, Xuechun Li, Ningning Li, Xiuxiu Xu, Jiazhang Xing, Hui Ge, Yi Xiao, Junchao Guo, Bin Wu, Xiaoyi Li, Liangrui Zhou, Lin Zhao, C. Bai, Qin Han, Zhao Sun, R. Zhao.(2023). POSTN Secretion by Extracellular Matrix Cancer-Associated Fibroblasts (eCAFs) Correlates with Poor ICB Response via Macrophage Chemotaxis Activation of Akt Signaling Pathway in Gastric Cancer.
[22] Weiqiang Huang, Longshan Zhang, Mi Yang, Xixi Wu, Xiaoqing Wang, Wenqi Huang, Lu Yuan, Hua Pan, Yin Wang, Zici Wang, Yuting Wu, Jihong Huang, Huazhen Liang, Shaoqun Li, Liwei Liao, Laiyu Liu, J. Guan.(2020). Cancer-associated fibroblasts promote the survival of irradiated nasopharyngeal carcinoma cells via the NF-κB pathway.

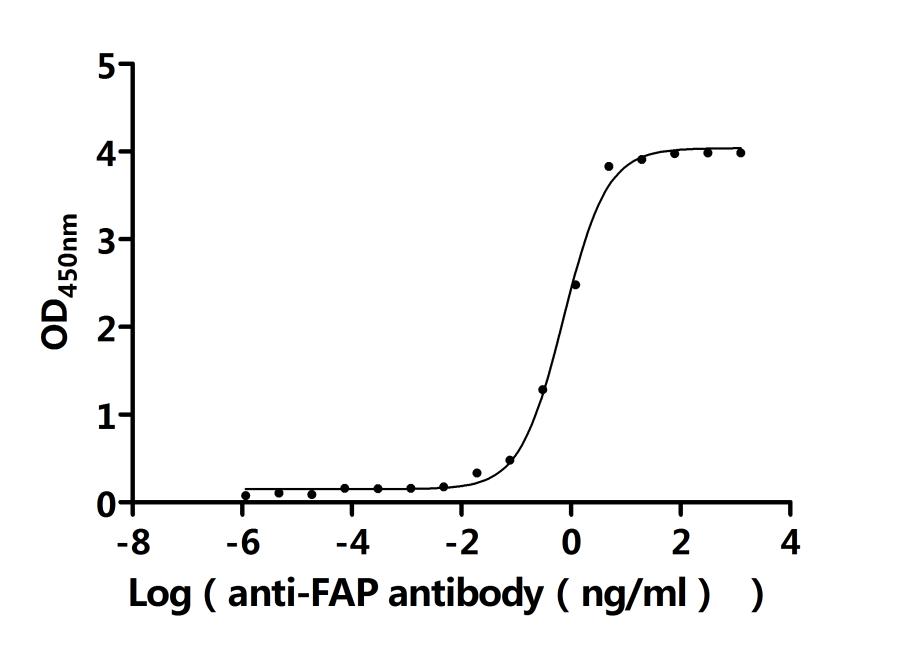
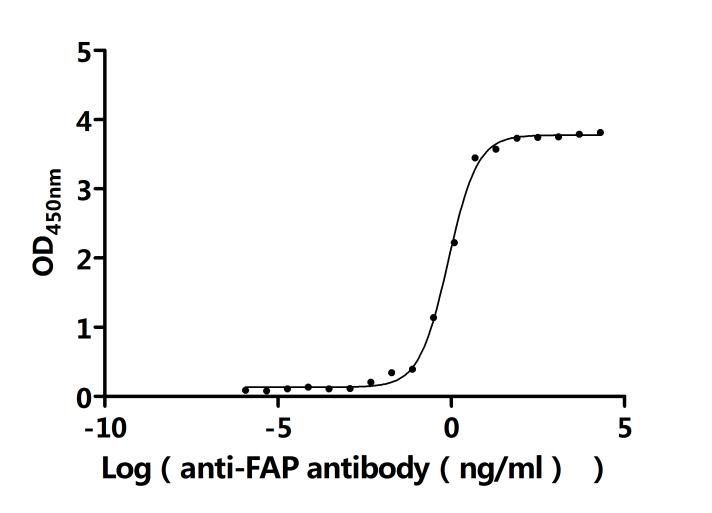
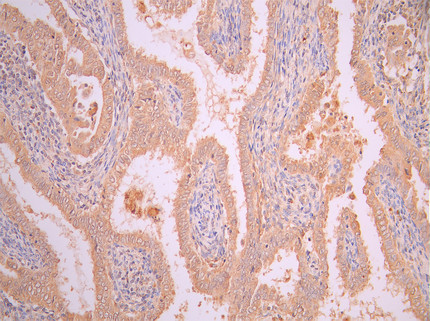
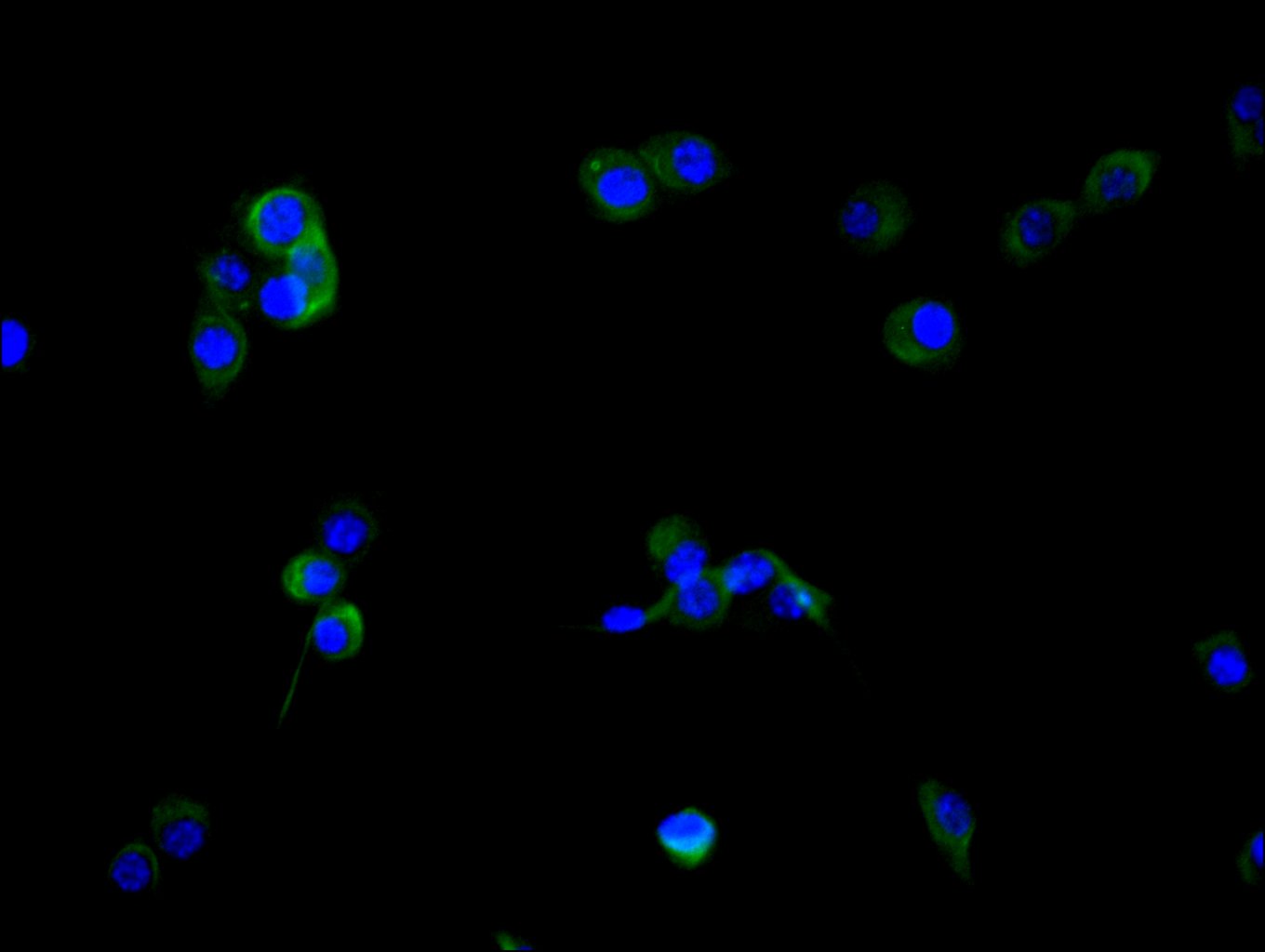



Comments
Leave a Comment