The thymic stromal lymphopoietin receptor (TSLPR, also known as CRLF2) is a key receptor subunit that mediates signaling by the epithelial-derived cytokine TSLP. Together with IL-7Rα, it forms a functional receptor complex. The TSLP/TSLPR pathway occupies a pivotal upstream position in the regulation of type 2 inflammatory responses. It not only contributes to the pathogenesis of immune-mediated diseases such as asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), and chronic obstructive pulmonary disease (COPD), but also drives disease progression in hematological malignancies like Ph-like acute lymphoblastic leukemia through its aberrant activation.
In recent years, as our understanding of the biological functions of TSLPR has deepened, this target has gradually emerged as an important bridge linking inflammation and cancer therapy. Among them, Verekitug (UPB-101), currently the world’s only TSLP receptor antagonist in clinical development, achieved a pivotal breakthrough in 2025, further highlighting the therapeutic potential of this target in chronic airway inflammatory diseases.
1. Structural and Functional Basis of CRLF2
CRLF2 (cytokine receptor-like factor 2), also known as the thymic stromal lymphopoietin receptor (TSLPR), is a member of the type I cytokine receptor superfamily. The gene encoding this protein is located in the pseudoautosomal region 1 (PAR1) of the X and Y chromosomes. CRLF2 itself lacks kinase activity and must form a heterodimer with the interleukin-7 receptor α chain (IL-7Rα) to jointly constitute the functional high-affinity receptor complex for thymic stromal lymphopoietin (TSLP) [1].
1.1 Protein domain composition
CRLF2 is a type I transmembrane glycoprotein composed of approximately 370 amino acids, featuring an N-terminal extracellular domain, a single transmembrane region, and a short C-terminal intracellular tail. The extracellular domain contains the hallmark WSX WS motif characteristic of the type I cytokine receptor family, which is crucial for maintaining the receptor’s conformation and facilitating ligand binding. However, compared to typical type I receptors, CRLF2 exhibits several distinctly atypical structural features: its extracellular domain lacks the second of four conserved cysteine residues, potentially impairing disulfide bond formation and overall folding; although the intracellular domain retains the Box1 motif—essential for JAK kinase anchoring—it lacks the canonical Box2 domain; furthermore, its intracellular tail contains only a single tyrosine residue located four positions upstream of the carboxyl terminus, and this site does not undergo phosphorylation upon ligand binding. These characteristics suggest that CRLF2 itself cannot independently carry out complete signal transduction and must rely on a co-receptor to provide functional compensation.
1.2 Assembly of the Receptor Complex and Ligand Recognition
CRLF2 itself cannot independently transmit signals; it must be co-expressed with IL-7Rα and form a heterodimer to achieve high-affinity binding to TSLP. TSLP first binds to the D1 domain of CRLF2, inducing a conformational change that subsequently recruits IL-7Rα, ultimately forming a stable ternary complex. This assembly mechanism not only enables high-affinity ligand recognition but also ensures spatiotemporal precision in signal activation, thereby preventing nonspecific immune activation.
2. The signaling pathways associated with CRLF2
2.1 Non-classical JAK-STAT activation mechanism
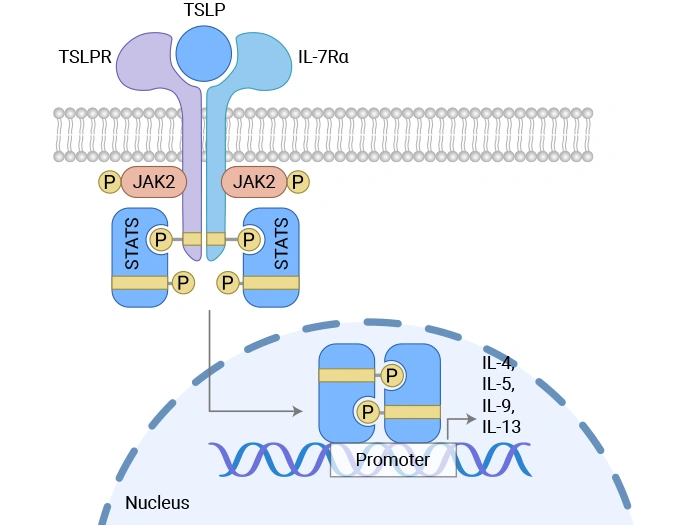
After TSLP binds to the CRLF2/IL-7Rα complex, it can trigger a downstream signaling cascade. The conventional view holds that this process involves trans-phosphorylation of JAK1 (which binds to IL-7Rα) and JAK2 (which binds to CRLF2), thereby activating STAT5. However, more detailed studies have revealed that TSLP-induced STAT5 activation does not rely on the canonical activity of JAK kinases—dominant-negative forms of JAK1 or JAK2 fail to block this process, whereas inhibition of Tec-family kinases partially attenuates STAT5 phosphorylation, suggesting that Tec kinases may play a critical role in this pathway. Moreover, the activation of STAT5 is not linearly coupled with its function of driving cell proliferation: STAT5 activation does not depend on the single intracellular tyrosine residue of CRLF2, yet this site is essential for cell proliferation.
2.2 Multi-pathway Cooperative Regulation of Cell Fate
In addition to STAT5, TSLP/CRLF2 signaling can also activate multiple parallel pathways. Src family kinases are involved in regulating cell proliferation; their inhibitors significantly block TSLP-induced proliferative responses without affecting STAT5 phosphorylation. Moreover, this pathway can also activate the MAPK/ERK and PI3K/AKT/mTOR cascades, which respectively regulate cell cycle progression and anti-apoptotic programs. Under pathological conditions—such as in Philadelphia chromosome–like acute lymphoblastic leukemia (Ph-like ALL)—aberrant expression or mutations of CRLF2 can lead to sustained activation of these signaling pathways, collectively driving the expansion and survival of leukemic clones.
Figure. Mechanism of thymic stromal lymphopoietin (TSLP)-induced signal transduction [2]
3. CRLF2 and Diseases
● CRLF2 and Ph-like Acute Lymphoblastic Leukemia
CRLF2 abnormalities represent one of the most common and clinically significant molecular features in Philadelphia chromosome–like acute lymphoblastic leukemia (Ph-like ALL). Ph-like ALL is a high-risk subtype of B-cell precursor acute lymphoblastic leukemia (BCP-ALL) whose gene expression profile resembles that of Philadelphia chromosome–positive (Ph+) ALL, yet it lacks the BCR-ABL1 fusion gene. Instead, it is characterized by various genetic alterations that activate kinase signaling pathways, among which CRLF2 rearrangements or overexpression occur most frequently [3]. Clinically, high CRLF2 expression is significantly associated with initial high white blood cell counts, poor minimal residual disease (MRD) clearance following induction therapy, increased risk of relapse, and reduced survival rates [4]. Currently, CRLF2 has been incorporated into international ALL treatment guidelines as a core diagnostic marker for Ph-like ALL and has emerged as an important breakthrough for targeted therapy.
● CRLF2 and Asthma
In asthma, particularly in severe eosinophilic or Th2-high asthma, airway epithelial cells secrete large amounts of TSLP following damage caused by environmental stimuli such as allergens, viruses, and pollutants. TSLP binds to the CRLF2/IL-7Rα complex on the surface of dendritic cells, type 2 innate lymphoid cells (ILC2s), and Th2 cells, thereby activating the STAT5/STAT3 signaling pathway and promoting the release of type 2 cytokines such as IL-4, IL-5, and IL-13. This, in turn, drives eosinophil infiltration, airway hyperresponsiveness, and excessive mucus production. Clinical studies have shown that bronchial biopsy tissues and serum from asthma patients exhibit significantly elevated levels of TSLP and CRLF2 expression, which correlate positively with disease severity [5].
● CRLF2 and Allergy
As a key upstream mediator in the allergic inflammatory cascade, TSLP drives type 2 immune responses by activating dendritic cells, Th2 cells, and group 2 innate lymphoid cells (ILC2s) through its receptor TSLPR (CRLF2). Studies have shown that ASP7266, a novel fully human monoclonal antibody targeting TSLPR, effectively blocks TSLP signaling, suppressing TSLP-induced cellular proliferation, release of the chemokine CCL17, Th2 cell differentiation, and IL-5 production from ILC2s. In a cynomolgus monkey model, ASP7266 completely inhibited allergic skin reactions and demonstrated preclinical pharmacological activity comparable to or potentially superior to that of tezepelumab, positioning it as a promising next-generation targeted therapeutic option for allergic diseases such as asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), and atopic dermatitis [6].
4. Progress in Research on CRLF2-Targeted Drugs
Currently, the pipeline of investigational drugs targeting TSLPR (CRLF2) encompasses a variety of drug types, including monoclonal antibodies, antibody-drug conjugates (ADCs), and bispecific antibodies (BsAbs). In the field of allergic inflammatory diseases, monoclonal antibodies that focus on blocking ligand-receptor interactions—such as Verekitug, which is in Phase 2 clinical trials—are making relatively rapid progress. In contrast, in the area of hematological malignancies—particularly Ph-like acute lymphoblastic leukemia—research and development are centered on leveraging novel technologies like antibody-drug conjugates and bispecific antibodies to achieve targeted cell killing. However, many of these ongoing projects remain at the preclinical exploration stage, and the overall translation process is still in its early phases.
| Drug |
Target |
Drug Type |
Indications in Development |
Developer |
Highest Development Stage |
| Verekitug |
TSLPR |
Monoclonal Antibody |
Chronic Obstructive Pulmonary Disease (COPD), Severe Asthma, Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) |
Upstream Bio, Inc. |
Clinical Phase II |
| AM E3-SG3249 |
TSLPR |
ADC |
Philadelphia chromosome–like Acute Lymphoblastic Leukemia (Ph-like ALL) |
IRBM SpA |
Preclinical |
| CRLF2-DM1 |
TSLPR x Tubulin |
ADC |
Philadelphia chromosome–like Acute Lymphoblastic Leukemia (Ph-like ALL) |
The University of New South Wales |
Preclinical |
| 1B7/CD3 BsAb(MD Anderson) |
CD3 x TSLPR |
Bispecific Antibody |
Acute Lymphoblastic Leukemia (ALL) |
The University of Texas MD Anderson Cancer Center |
Preclinical |
| Anti TSLP antibody |
TSLPR |
Monoclonal Antibody |
Autoimmune Diseases |
China Resources Biopharma Co., Ltd. |
Preclinical |
(Data as of November 20, 2025, sourced from Synapse)
References
[1] Liu Y J, Soumelis V, Watanabe N, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007; 25: 193-219.
[2] Ebina-Shibuya R,Leonard W J. Role of thymic stromal lymphopoietin in allergy and beyond. Nat Rev Immunol. 2023;23 (1):24-37.
[3] Potter N, Jones L, Blair H, et al. Single-cell analysis identifies CRLF2 rearrangements as both early and late events in Down syndrome and non-Down syndrome acute lymphoblastic leukaemia. Leukemia. 2019;33 (4):893-904.
[4] Jiang M, Zou X, Lu L. Potential efficacy and prognosis of silencing the CRLF2‑mediated AKT/mTOR pathway in pediatric acute B‑cell lymphoblastic leukemia. Oncol Rep. 2019;41 (2):885-894.
[5] Malik B, McKerrow R, Harrington J, et al. ILC2 cells from severe allergic and eosinophilic asthma demonstrate increased expression of TSLP receptor (TSLPR) and enhanced proliferative capacity. Eur Respir J. 2021;58(suppl 65):PA823.
[6] Numazaki M, Abe M, Hanaoka K, et al. ASP7266, a Novel Antibody against Human Thymic Stromal Lymphopoietin Receptor for the Treatment of Allergic Diseases. J Pharmacol Exp Ther. 2022;380 (1):26-33.
CUSABIO team. CRLF2 (TSLPR): A Key Target Linking Type 2 Inflammation to Hematologic Malignancies. https://www.cusabio.com/c-21281.html


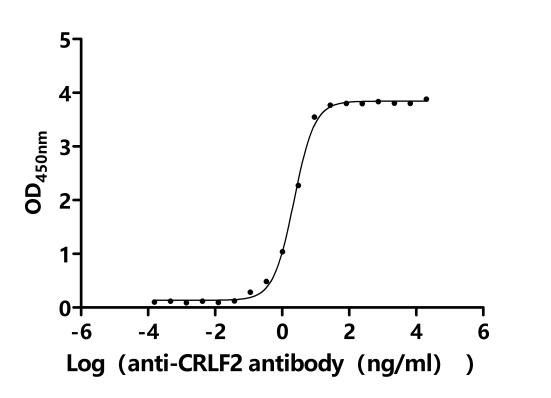



Comments
Leave a Comment