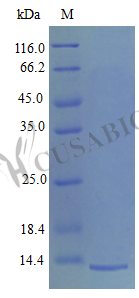
Recombinant Mouse Platelet factor 4 protein (Pf4) is produced in E. coli and represents the full length of the mature protein, spanning amino acids 30-105. This tag-free protein reaches purification levels greater than 97% as determined by SDS-PAGE analysis. It maintains biological activity, which appears to be confirmed by a chemotaxis bioassay using human neutrophils within a concentration range of 10-100 ng/ml. The endotoxin level remains strictly controlled, staying below 1.0 EU/µg as verified by the LAL method.
Platelet factor 4 (Pf4) is a chemokine mainly released by platelets during coagulation. It likely plays a crucial role in modulating the immune response by influencing neutrophil activity and other leukocyte functions. Pf4 appears to be involved in various biological pathways, including inflammation and wound healing processes. Its significance in research stems from its involvement in these critical physiological and pathological pathways, making it an essential protein for investigation in immunological studies.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Neutrophil Chemotaxis Assays and Migration Studies
This recombinant mouse PF4 is confirmed to be biologically active in human neutrophil chemotaxis (10-100 ng/ml) and suitable as a positive control in migration studies. However, researchers should validate cross-reactivity in mouse neutrophil systems to ensure physiological relevance, as species-specific differences in PF4-receptor interactions may exist. The high purity and low endotoxin support reliable results, but optimal concentrations may need adjustment for primary mouse neutrophils.
2. Comparative Protein Structure-Function Analysis
The tag-free, high-purity PF4 is appropriate for comparative studies with human PF4. However, the demonstrated activity in human cells rather than mouse cells may complicate direct functional comparisons. Researchers should perform parallel assays in both human and mouse systems to properly interpret species-specific differences, noting that glycosaminoglycan binding properties may vary between species.
3. Antibody Development and Validation
This PF4 serves as a good antigen for antibody development, but researchers should validate that antibodies generated against this recombinant protein recognize native mouse PF4 from platelet sources. The confirmed cross-species bioactivity suggests conserved epitopes, but species-specific validation is essential for reliable immunological applications.
4. Heparin-Binding and Glycosaminoglycan Interaction Studies
The protein is suitable for glycosaminoglycan binding studies, but researchers should note that mouse PF4 may have different heparin-binding affinity compared to human PF4. The high purity supports accurate binding measurements, but species-specific binding characteristics should be determined empirically rather than assumed from human PF4 data.
5. Preclinical Research Model Development
This mouse PF4 can be used in preclinical studies, but the human neutrophil activity data may not directly translate to mouse models. Researchers should establish dose-response relationships in mouse-specific systems and validate that the recombinant protein elicits expected physiological responses comparable to endogenous PF4 in murine models.
Final Recommendation & Action Plan
This recombinant mouse PF4 is a valuable tool with demonstrated cross-species bioactivity, but its applications require species-specific validation. First, establish mouse-specific activity profiles using primary mouse neutrophils and in vivo models to confirm physiological relevance. For chemotaxis studies, use the 10-100 ng/ml range as a starting point but optimize for mouse systems. When developing antibodies, validate cross-reactivity with native platelet-derived PF4. For binding studies, determine mouse-specific binding parameters rather than relying on human PF4 data. While the protein's high purity and low endotoxin make it suitable for sensitive assays, always include appropriate controls for species-specific responses. The cross-reactivity with human cells is valuable for translational studies but requires careful interpretation when applied to mouse-specific research questions.