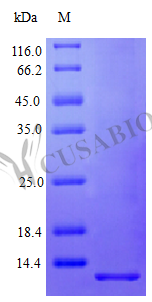
Recombinant Human Pro-neuregulin-1, membrane-bound isoform protein (NRG1) is expressed in E. coli, specifically covering the 177-237 amino acid region of Isoform 12. This tag-free product achieves a purity exceeding 96% as verified by SDS-PAGE and maintains an endotoxin level under 1.0 EU/µg, as determined by the LAL method. The protein appears to be fully biologically active, with an ED50 of less than 50 ng/ml in a cell proliferation assay using serum-free human MCF-7 cells, demonstrating a specific activity greater than 2.0 × 10^4 IU/mg.
Pro-neuregulin-1 seems to play a crucial role in cellular communication and growth, particularly in the nervous system. It's involved in various pathways related to cell proliferation and differentiation. This protein may be important for research because it likely contributes to understanding processes like synaptic plasticity and neural development. Many researchers find it a valuable tool in studies focused on cellular signaling and neurobiology.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Cell Proliferation and Growth Factor Studies
This recombinant NRG1 fragment (177-237aa) is highly biologically active (ED₅₀ < 5 ng/ml in MCF-7 cells) and suitable for proliferation studies. However, the partial sequence represents only the EGF-like domain of isoform 12 and may not fully recapitulate the signaling complexity of full-length NRG1. Researchers should validate that proliferation kinetics and ErbB receptor activation match those induced by full-length NRG1 in their specific cell models. The high purity (>96%) supports reliable results, but the fragment's activity may be limited to specific ErbB receptor dimers (e.g., ErbB4 homodimers).
2. ErbB Receptor Binding and Signaling Assays
The protein is appropriate for ErbB receptor binding studies, as the 177-237aa region contains the core EGF-like domain responsible for receptor interaction. However, the partial nature may alter receptor dimerization preferences compared to full-length NRG1. Binding assays should validate affinity for specific ErbB receptors (ErbB3/ErbB4) and compare signaling outcomes (e.g., AKT/ERK phosphorylation) with full-length protein to ensure physiological relevance.
3. Structure-Function Relationship Studies
This fragment is valuable for studying the EGF-like domain's structure-function relationships, but it cannot inform about the roles of other NRG1 domains (e.g., transmembrane or cytoplasmic regions). Mutagenesis studies should focus on receptor-binding residues, and conclusions should be contextualized as specific to this domain. The tag-free design supports authentic interactions, but the E. coli expression lacks mammalian post-translational modifications that may affect structure.
4. Antibody Development and Validation
This high-purity NRG1 fragment serves as a good immunogen for domain-specific antibodies, but antibodies will only recognize epitopes within the 177-237aa region. Comprehensive validation should include testing against full-length NRG1 to ensure recognition of native protein forms. The confirmed bioactivity supports the development of function-blocking antibodies targeting the receptor-binding domain.
5. Pharmacological Research Tools
The protein is suitable for screening NRG1 pathway modulators, but the partial sequence may not reflect the full-length NRG1's pharmacological profile. Hit compounds should be validated against full-length protein or native NRG1 signaling in relevant physiological models. The high potency (ED₅₀ < 5 ng/ml) supports sensitive assays, but researchers should account for potential differences in compound effects on fragment versus full-length protein.
Final Recommendation & Action Plan
This recombinant human NRG1 partial protein (177-237aa) demonstrates high biological activity and is suitable for ErbB receptor-focused studies, but its partial nature requires careful validation. First, confirm that key functions—receptor binding specificity, signaling amplitude, and proliferation effects—match full-length NRG1 in your experimental systems. For cell-based assays, the high potency allows for low working concentrations (1-10 ng/ml), but optimization for each cell type, as ErbB receptor expression varies. When developing antibodies, this fragment is ideal for generating domain-specific reagents, but supplemented with full-length antigen for comprehensive coverage. The E. coli expression produces a non-glycosylated protein, which is acceptable as the EGF-like domain is minimally glycosylated, but critical findings should be verified with mammalian-expressed NRG1 when studying complex biological contexts. Always include appropriate controls and consider that the fragment may exhibit different stability and receptor engagement kinetics compared to full-length NRG1.