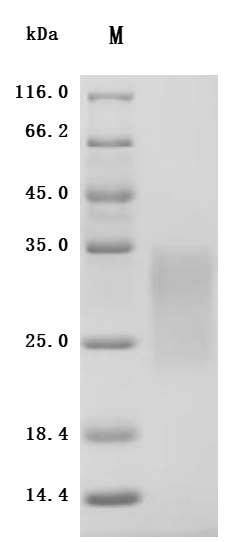
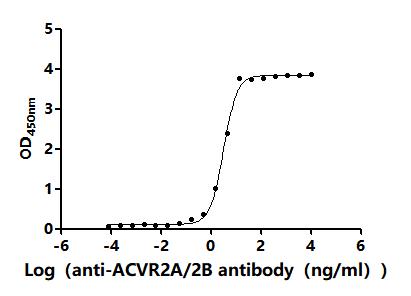
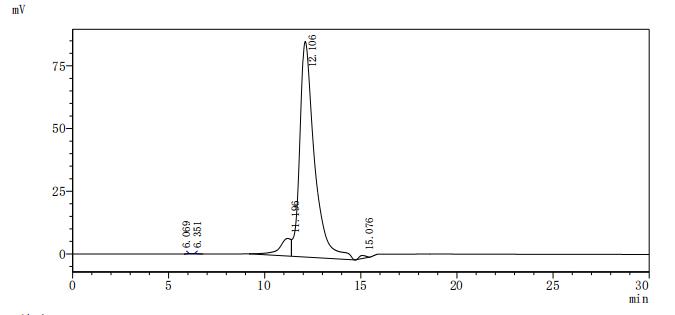
The recombinant human ACVR2B protein is expressed from HEK293 cells with a 10xHis tag at the C-terminus. It contains Ser19-Thr137 of the human ACVR2B protein. Its purity is up to 95% as determined by SDS-PAGE and SEC-HPLC. Its biological activity is confirmed in a functional ELISA, where immobilized human ACVR2B at 2 μg/mL can bind the anti-ACVR2B recombinant antibody (CSB-RA623829MA1HU), with the EC50 of 2.983-3.438 ng/mL.
The human ACVR2B protein plays a critical role in various biological processes, primarily through its involvement in the TGF-β signaling pathway. This receptor is essential for mediating the effects of several ligands, including activins and myostatin, which are pivotal in regulating muscle growth and differentiation, as well as other physiological functions.
ACVR2B mainly regulates muscle mass. It acts as a receptor for myostatin, a negative regulator of muscle growth. Studies have shown that ACVR2B binds myostatin with high affinity, and its inhibition can lead to significant increases in muscle mass, comparable to the effects observed in myostatin knockout models [1][2]. The blockade of ACVR2B signaling has been demonstrated to preserve skeletal muscle in various contexts, including cancer cachexia and disuse atrophy [3][4][5]. Specifically, the use of soluble forms of ACVR2B, such as ACVR2B-Fc, has shown promise in counteracting muscle wasting induced by chemotherapy [5][6].
ACVR2B is also implicated in bone metabolism. It has been identified as a high-affinity receptor for activin A and bone morphogenetic proteins (BMPs), which are crucial for osteogenic differentiation [7][8]. The ACVR2B signaling pathway can inhibit osteogenic differentiation, indicating that ACVR2B plays a dual role in both muscle and bone homeostasis [7][8]. Inactivation of ACVR2B has been proposed as a therapeutic target for conditions characterized by muscle and bone loss, such as osteoporosis and cachexia [6][7].
Moreover, ACVR2B is involved in immune responses and tissue remodeling. Activin A, which signals through ACVR2B, has been linked to various immune functions, including macrophage polarization and responses in allergic diseases [8][9].
References:
[1] S. Lee, A. Lehar, C. Ly, Q. Pham, M. Michaud, R. Rydzik, et al. Functional redundancy of type i and type ii receptors in the regulation of skeletal muscle growth by myostatin and activin a, Proceedings of the National Academy of Sciences, vol. 117, no. 49, p. 30907-30917, 2020. https://doi.org/10.1073/pnas.2019263117
[2] A. Windelinckx, G. Mars, W. Huygens, M. Peeters, B. Vincent, C. Wijmenga, et al. Comprehensive fine mapping of chr12q12-14 and follow-up replication identify activin receptor 1b (acvr1b) as a muscle strength gene, European Journal of Human Genetics, vol. 19, no. 2, p. 208-215, 2010. https://doi.org/10.1038/ejhg.2010.173
[3] T. Li, R. Wu, Y. Zhang, & D. Zhu. A systematic analysis of the skeletal muscle mirna transcriptome of chicken varieties with divergent skeletal muscle growth identifies novel mirnas and differentially expressed mirnas, BMC Genomics, vol. 12, no. 1, 2011. https://doi.org/10.1186/1471-2164-12-186
[4] K. Murphy, V. Cobani, J. Ryall, C. Ibebunjo, & G. Lynch. Acute antibody-directed myostatin inhibition attenuates disuse muscle atrophy and weakness in mice, Journal of Applied Physiology, vol. 110, no. 4, p. 1065-1072, 2011. https://doi.org/10.1152/japplphysiol.01183.2010
[5] T. Nissinen, J. Degerman, M. Räsänen, A. Poikonen, S. Koskinen, E. Mervaala, et al. Systemic blockade of acvr2b ligands prevents chemotherapy-induced muscle wasting by restoring muscle protein synthesis without affecting oxidative capacity or atrogenes, Scientific Reports, vol. 6, no. 1, 2016. https://doi.org/10.1038/srep32695
[6] R. Barreto, Y. Kitase, T. Matsumoto, F. Pin, K. Colston, K. Couch, et al. Acvr2b/fc counteracts chemotherapy-induced loss of muscle and bone mass, Scientific Reports, vol. 7, no. 1, 2017. https://doi.org/10.1038/s41598-017-15040-1
[7] L. Barrault, J. Gide, T. Qing, L. Lesueur, J. Tost, J. Denis, et al. Expression of mirnas from the imprinted dlk1/dio3 locus signals the osteogenic potential of human pluripotent stem cells, Cells, vol. 8, no. 12, p. 1523, 2019. https://doi.org/10.3390/cells8121523
[8] O. Olsen, K. Wader, H. Hella, A. Mylin, I. Turesson, I. Nesthus, et al. Activin a inhibits bmp-signaling by binding acvr2a and acvr2b, Cell Communication and Signaling, vol. 13, no. 1, 2015. https://doi.org/10.1186/s12964-015-0104-z
[9] K. Tengvall, E. Sundström, C. Wang, K. Bergvall, O. Wallerman, E. Pederson, et al. Bayesian model and selection signature analyses reveal risk factors for canine atopic dermatitis, Communications Biology, vol. 5, no. 1, 2022. https://doi.org/10.1038/s42003-022-04279-8