Transforming growth factor β signaling pathway is a series of signal transduction processes mediated by transforming growth factor. TGF-β signaling pathway plays a key role in cell proliferation, interstitial production, differentiation, apoptosis, embryonic development, organ formation, immune function and inflammatory response.
1. The process of TGF-β signaling pathway
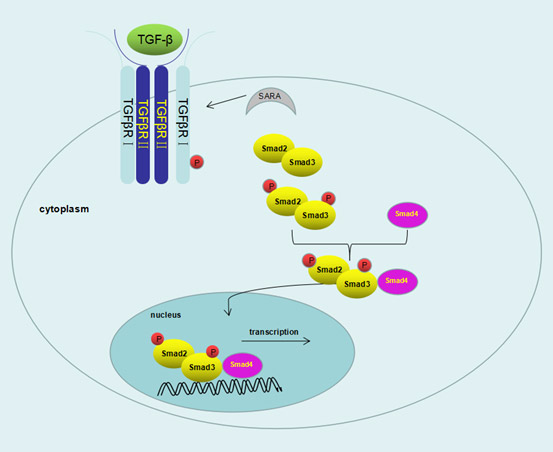
First, TβRⅡneeds to be activated by self-phosphorylated in its amino acid residues Ser213 and Ser409 to be activated, then it interacts with TβRⅠ and activates TβRⅠ[1]. After that, Tyrosine residue phosphorylationcould also occur in TβRⅠ[2]. In the absence of type Ⅱ receptor, type I receptor could not bind to TGF-β independently. The GS functional domain (a highly conserved domain of glycine and serine residues) of type I receptor was phosphorylated by activated type Ⅱ receptor. This region plays an important role in the activation of TGF-βRⅠ kinase. The activated type I receptor can phosphorylate its downstream signal-just like activated Smad2 and Smad3. Smad2 and Smad3 were recruited to type I receptor by SARA(smad-anchor for receptor activation). The phosphorylated Smad2 and Smad3 then form a trimer complexes with Smad4. This complex can enter the nucleus and bind to the region of Smad-binding element with the help of DNA-binding auxiliaries and then induce transcription. This complex can enter the nucleus and bind to the region of Smad-binding element with the help of DNA-binding auxiliaries to induce transcription, so then regulating cell proliferation, differentiation, migration and apoptosis. After transcription, the Smad complex can be dissociated, and phosphorylated R-Smads are dephosphorized by phosphatase in the nucleus (e.g. PPM1A / PP2C), so that these R-Smads molecules are returned to the cytoplasm to form a "Smad loop"[3].
Factors affecting the TGF-β 1/Smads signaling pathway
In organisms, The TGF-β signaling pathway in organisms is controlled by a variety of factors, such as microenvironmental conditions[4][5], cytokines[6] and growth factors[7], microRNAs (MiRNAs)[8], long non-coding RNA[9], phosphorylated and dephosphorylated kinase[3], ubiquitin ligase and diubiquitin enzyme[10] and other factors.
TGF-β receptor: TGF-β superfamily receptors are mainly composed of three subtypes of TGF-βRⅡ and TGF-βRⅢ receptors, which including extracellular, transmembrane and intracellular domains. The heteropolymer composed of two TβRⅠ and two TβRⅡ molecules contains functional receptor. TGF-β type Ⅲ receptor is an auxiliary receptor and is not directly involved in signal transduction. The main function of TGF-β is to increase the binding ability of TGF-β on cell surface and to supply TGF-β to type Ⅰ and Ⅱ receptors[11]. TβR III can also inhibit the metastasis, invasion, growth and angiogenesis of tumor cells,it has potential application value in tumor therapy[12].
Smads: Smads is an important signal transduction and regulation molecule in cells, which can directly transfer TGF-β signal from the cell membrane into the nucleus. Receptor-regulated Smad (R-Smads) is a direct substrate of receptor kinases type Ⅰ, which is related to the specificity of signal transduction pathway; common-mediator Smad-β (Co-Smad) is necessary for the translocation of all TGF-β signals into the nucleus; The signal transduction suppressive Smads (I-Smads) involved in all TGF-β superfamily members is a negative regulator of TGF-β/Smad signal transduction pathway. It inhibits the phosphorylation of R-Smads by binding to the activated TβR-Ⅰ receptor and thus blocks the signal pathway and Blocking the biological effect of TGF-β[13].
Thrombospondin S1 (THB-S1): THB-S1 induces platelet aggregation and inhibits angiogenesis. It is an important activator of TGF-β in wound healing, cell adhesion, migration, proliferation and differentiation. It can change the conformation of TGF-β, expose its binding site to cell receptor and then activate TGF-β signaling pathway.
S-phase kinase associated protein 1 (s-phase kinase association protein 1/SKP1): a multifunctional protein involved in cell cycle regulation. SKP1 can also be used as downstream regulator of TGF-β/Smads signaling pathway for Ubiquitin degradation of related substances. As a downstream regulation factor in TGF-β/Smads signaling pathway, SKP1 regulates follicle formation and ovulation in mammals.
Other related factors: the TGF-β 1/Smads signaling pathway has extensive communication with other signaling pathways. For example, epidermal growth factor (EGF), lipopolysaccharide, tumor necrosis factor (TNF), and interleukin -1 beta (IL-1 beta) can induce Samd7 production.
In addition, TGF-β signal can interact with Wnt signal, such as P38. Different pathways form a complex regulatory system, which can effectively regulate the normal operation of TGF-β signaling pathway and endue TGF-β complex and diverse biological effects.
2. The members of TGF-β signaling pathway
The TGF-β signaling pathway involves receptors and some signal transduction molecules, as shown in the following table1:
Table 1 TGF-β signaling pathway Components
|
TGF β superfamily ligand
|
Type II Receptor
|
Type I receptor
|
R-SMADs
|
coSMAD
|
Ligand inhibitors
|
|
Activin A
|
ACVR2A
|
ACVR1B (ALK4)
|
SMAD2, SMAD3
|
SMAD4
|
Follistatin
|
|
GDF1
|
ACVR2A
|
ACVR1B (ALK4)
|
SMAD2,SMAD3
|
SMAD4
|
unknown
|
|
GDF11
|
ACVR2B
|
ACVR1B (ALK4), TGFβRI (ALK5)
|
SMAD2,SMAD3
|
SMAD4
|
unknown
|
|
Nodal
|
ACVR2B
|
ACVR1B (ALK4), ACVR1C (ALK7)
|
SMAD2, SMAD3
|
SMAD4
|
Lefty
|
|
Bone morphogenetic proteins
|
BMPR2
|
BMPR1A (ALK3), BMPR1B (ALK6)
|
SMAD1,SMAD5,SMAD8
|
SMAD4
|
Noggin, Chordin, DAN
|
|
TGFβs
|
TGFβRII
|
TGFβRI (ALK5)
|
SMAD2, SMAD3
|
SMAD4
|
LTBP1, THBS1, Decorin
|
Content of the Table 1 is derived from Wikipedia
3. The function of TGF-β signaling pathway
TGF-β plays a key role in the growth, development and differentiation of cells and tissues [14] and plays an important regulatory role in cell proliferation, interstitial production, differentiation, apoptosis, embryonic development, organ formation, immune function, inflammatory response and wound repair.
The immune function of TGF-β is mainly reflected in immunosuppression, TGF-β can inhibit the proliferation and differentiation of T and B lymphocytes. TGF-β can stimulate and inhibit the proliferation of cells, which depends on the type of cell and the state of differentiation. For example, TGF-β can promote the mitosis of osteoblasts and inhibit the growth of hepatocytes. In addition, TGF-β has a certain regulatory effect on cell adhesion.
4. Diseases associated with TGF-β signaling pathway
Abnormal TGF-β expression and signal transduction are associated with the development of many diseases, such as cancer, fibrosis, hereditary hemorrhagic capillary dilatation and family primary pulmonary hypertension [15].
TGF-β and tumor:
TGF-β is associated with tumor development, progression and metastasis. At the early stage of tumorigenesis, TGF-β inhibits the growth of tumor cells [16], but in the middle and late stages of the tumor, the effect of TGF-β on tumor was mainly manifested as promoting tumor progression. TGF-β also promotes tumor growth and metastasis. A large number of studies have found that TGF-β/Smad signaling pathway plays an important role in the pathogenesis of airway neoplasms, as well as in the pathogenesis of diabetic nephropathy (DN), mainly by inducing the accumulation of extracellular matrix (ECM) in glomerular and tubular cells.
TGF-β plays a key role in the process of fibrosis:
The increased expression of transforming growth factor-β is a common pathway of organic fibrosis and can be used as a target of treatment[17]. Only subcutaneous injection of TGF-β induced collagen deposition and fibrosis in rats, TGF-β mRNA expression was also found in glomeruli of diabetic rats[18].
Relationship between TGF-β and ocular diseases:
TGF-β plays an important role in the occurrence and development of cataract and can directly affect the occurrence and development of glaucoma. With the further study of the relationship between transforming growth factor β (TGF-β) and various diseases, people will have a deeper understanding of the role of TGF-β, which will be helpful to the diagnosis and treatment of various diseases.
5. Related signaling pathways
TGF-β/Smad signaling pathway is the main pathway for TGF-β to play a biological role [19]. In addition to the classical TGF-β/Smad signaling pathway, there are some non-Smad pathways. TGF-β can activate MAPK signaling pathways such as Rho like GTPase pathway and PI3K / Akt signal pathway. TGF-β not only activates MAPK signal pathway through RTK/Ras/ERK, but also activates MAPK signal transduction process by TRAF6-TAK1-JNK-p38. Activated MAPK reactivates a series of protein molecules (mainly transcription factors in the nucleus), which play an important role in cell survival, differentiation, proliferation and apoptosis. In addition, TGF-β activates the Rho like GTPase signaling pathway through Par6-Smurf1-RhoA, or activates the PI3K/AKT/mTOR signaling pathway, It plays a certain role in epithelium to mesenchymal transition, fibroblast proliferation and morphological changes.
6. The lastest research of TGF-β signaling pathway
At present, the research on TGF-β is mainly focused on its effect and regulation on different diseases, and the interaction between different factors and TGF-β. In recent years, many cytokines related to cardiovascular diseases have been found and studied. TGF-β/Smads signaling pathway is one of them. The signal pathway of TGF-β/Smads plays an important role in the progression of myocardial fibrosis and is thought to be closely related to the pathological process of fibrosis in various tissues and organs. Transforming growth factor β and bone morphogenetic protein (BMPs) pathway can also regulate the abnormal proliferation of pulmonary artery vascular smooth muscle cells, TGF-β and BMP signaling pathway have antagonistic effects on the proliferation of vascular smooth muscle cells[20][21].
In recent years, more and more studies have found[22][23] that integrin and TGF-β are related to each other in many diseases, For example, TGF-β induces the expression of integrin in fibrotic diseases, and the activation of integrin can enhance the role of TGF-β in collagen synthesis.
References
[1] Luo K, Lodish HF. Positive and negative regulation of type II TGF-β receptor signal transduction by autophosphorylation on multiple serine residues [J]. EMBO J, 1997, 16(8): 1970-1981.
[2] Lee MK, Pardoux C, Hall MC, et al. TGF-β activates Erk MAP kinase signalling through direct phosphorylation of ShcA [J]. EMBO J, 2007, 26(17): 3957-3967.
[3] Lin X, Duan X, Liang Y Y, et al. PPM1A Functions as a Smad Phosphatase to Terminate TGFβ Signaling [J]. Cell, 2006, 125(5): 915-928.
[4] Ma Biao, Cheng Hongcheng, Gao Ruize, et al. Zyxin-Siah2-Lats2 axis mediates cooperation between Hippo and TGF-β signalling pathways [J]. Nature Communications, 2016, 7: 11123.
[5] Barouch D H, Ghneim K, Bosche W J, et al. Rapid Inflammasome Activation following Mucosal SIV Infection of Rhesus Monkeys [J]. Cell, 2016, 165(3): 656-667.
[6] Alonso-Merino E, Martín O R, Ruíz-Llorente L, et al. Thyroid hormones inhibit TGF-β signaling and attenuate fibrotic responses [J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(24): E3451.
[7] Demagny H, Araki T, Derobertis E. The Tumor Suppressor Smad4/DPC4 Is Regulated by Phosphorylations that Integrate FGF, Wnt, and TGF-β Signaling [J]. Cell Reports, 2014, 9(2): 688-700.
[8] Yin S, Fan Y, Zhang H, et al. Differential TGF-β pathway targeting by miR-122 in humans and mice affects liver cancer metastasis [J]. Nature Communications, 2016, 7, 11012.
[9] Zhao J J, Hao S, Wang L L, et al. Long non-coding RNA ANRIL promotes the invasion and metastasis of thyroid cancer cells through TGF-β/Smad signaling pathway [J]. Oncotarget, 2016, 7, 57903–57918.
[10] Dupont S, Mamidi A, Cordenonsi M, et al. FAM/USP9x, a deubiquitinating enzyme essential for TGF β signaling, controls Smad4 monoubiquitination [J]. Cell, 2009, 136, 123–135.
[11] Taylor AW. Review of the activation of TGF-β in immunity [J]. J Leukoc Biol, 2009, 85(1): 29-33.
[12] Gatza1 CE, Oh SY, Blobe GC. Roles for the type Ⅲ TGF-β receptor in human cancer [J]. Cell Signal, 2010, 22(8): 1163-1174.
[13] Dennler S, Goumans M J, Ten D P. Transforming growth factor beta signal transduction [J]. J Leukoc Biol, 2002, 71(5): 731-740.
[14] Ji F, Fu S J, Shen S L, et al. The prognostic value of combined TGF-β1 and ELF in hepatocellular carcinoma [J]. Bmc Cancer, 2015, 15(1): 116.
[15] Schmierer B, Hill CS. TGF β-SMAD signal transduction: molecular specificity and functional flexibility [J]. Nat Rev Mol Cell Biol, 2007, 8(12): 970 -982.
[16] Pinkas J, Teicher B A. Role of TGF-β in Tumor Progression and Metastasis [M]. Cancer Drug Resistance. Humana Press, 2006: 469-489.
[17] Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor TGF-β signaling in cardiac remodeling [J]. J Mol Cell Cardiol, 2011, 51(4): 600-606.
[18] Wull, Cox A, Roe CJ, et al. Transforming growth factor beta and Renal injury following subtotal nephrectomy in the rat: role of the Renin-angiotensin system [J]. Kidney Int, 1997; 51: 1553.
[19] Hata A, Chen YG. TGF-β Signaling from Receptors to Smads [J]. Cold Spring Harb Perspect Biol 2016; 8.
[20] Morrell NW,Yang X,Upton PD,et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor -β1 and bone morphogenetic proteins [J]. Circulation, 2001, 104(7): 790-795.
[21] Sheares KK, Jeffery TK, Long L, et al. Differential effects of TGF-β 1 and BMP-4 on the hypoxic induction of cyclooxygenase-2 in human pulmonary artery smooth muscle cells [J]. Am J Physiol Lung Cell Mol Physiol, 2004, 287(5): L919-927.
[22] Hayashida T, Jones JC, Lee CK, et al. Loss of beta1-integrin enhances TGF-β 1-induced collagen expression in epithelial cells via increased alphavbeta3-integrin and Rac1 activity [J].J Biol Chem, 2010, 285(40): 30741-30751.
[23] Girgert R, Martin M, Kruegel J, et al. Integrin alpha2-deficient mice provide insights into specific functions of collagen receptors in the kidney [J]. Fibrogenesis Tissue Repair, 2010, 3: 19.
CUSABIO team. The overview of TGF-β signaling pathway. https://www.cusabio.com/c-20712.html





Comments
Leave a Comment