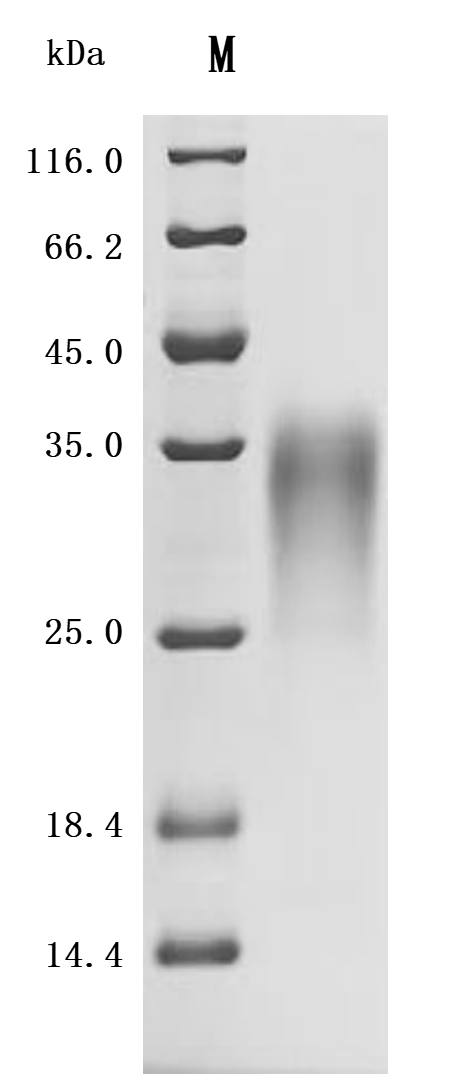
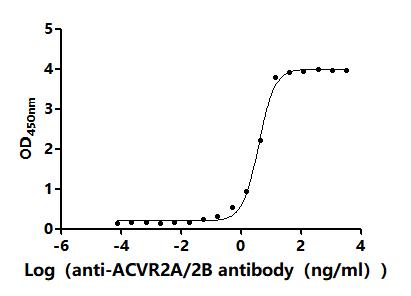
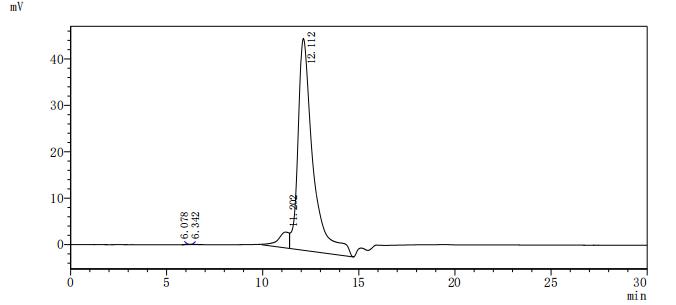
The recombinant mouse ACVR2B protein is co-expressed with a C-terminal 10*his-tag in mammalian cells. Its expression region encodes the 19-137aa of the mouse ACVR2B protein. It contains low endotoxin less than 1.0 EU/ug as measured by the LAL method. Its activity is validated through a functional ELISA where immobilized mouse ACVR2B at 2 μg/mL can bind the anti-ACVR2A&ACVR2B recombinant antibody (CSB-RA623829MA1HU), with the EC50 of 3.560-4.235 ng/mL.
The mouse ACVR2B protein is a member of the TGF-β superfamily of receptors. It plays a crucial role in various biological processes, particularly in muscle growth regulation and the response to muscle wasting conditions. ACVR2B functions primarily as a receptor for myostatin and other activins, which are negative regulators of muscle growth. The inhibition of ACVR2B signaling has been shown to lead to increased muscle mass, a phenomenon often referred to as double muscling in animals, including mice [1][2].
In the context of muscle physiology, ACVR2B is involved in the signaling pathways that mediate muscle atrophy and hypertrophy. Studies have demonstrated that transgenic mice expressing a dominant negative form of ACVR2B exhibit significant muscle hypertrophy due to the blockade of myostatin signaling [2][3]. The ACVR2B's activity is also critical in other systems, such as the heart, where its signaling is implicated in cardiac development and response to stress [4].
Moreover, ACVR2B has been implicated in pathological conditions such as cachexia, a syndrome characterized by muscle wasting often associated with cancer and other chronic diseases. Research indicates that blocking ACVR2B ligands can restore muscle protein synthesis and mitigate muscle loss in cachexia models [5][6]. The systemic administration of soluble forms of ACVR2B has been shown to improve muscle mass and function in various experimental settings, highlighting its potential as a therapeutic target for muscle-wasting disorders [7].
ACVR2B is also involved in developmental processes. Knockout studies have revealed that ACVR2B signaling is essential for proper vertebral development and overall embryonic growth, indicating its broader significance in developmental biology [8]. The receptor's interactions with various ligands, including activins and growth differentiation factors, further underscore its multifaceted role in cellular signaling and tissue homeostasis [9].
References:
[1] Y. Klimentidis, J. Bea, P. Thompson, W. Klimecki, C. Hu, G. Wu, et al. Genetic variant in acvr2b is associated with lean mass, Medicine & Science in Sports & Exercise, vol. 48, no. 7, p. 1270-1275, 2016. https://doi.org/10.1249/mss.0000000000000889
[2] S. Lee. Quadrupling muscle mass in mice by targeting tgf-ß signaling pathways, Plos One, vol. 2, no. 8, p. e789, 2007. https://doi.org/10.1371/journal.pone.0000789
[3] S. Lee, A. Lehar, J. Meir, C. Koch, A. Morgan, L. Warren, et al. Targeting myostatin/activin a protects against skeletal muscle and bone loss during spaceflight, Proceedings of the National Academy of Sciences, vol. 117, no. 38, p. 23942-23951, 2020. https://doi.org/10.1073/pnas.2014716117
[4] J. Hulmi, T. Nissinen, M. Räsänen, J. Degerman, J. Lautaoja, K. Hemanthakumar, et al. Prevention of chemotherapy‐induced cachexia by acvr2b ligand blocking has different effects on heart and skeletal muscle, Journal of Cachexia Sarcopenia and Muscle, vol. 9, no. 2, p. 417-432, 2017. https://doi.org/10.1002/jcsm.12265
[5] T. Nissinen, J. Hentilä, F. Penna, A. Lampinen, J. Lautaoja, V. Fachada, et al. Treating cachexia using soluble acvr2b improves survival, alters mtor localization, and attenuates liver and spleen responses, Journal of Cachexia Sarcopenia and Muscle, vol. 9, no. 3, p. 514-529, 2018. https://doi.org/10.1002/jcsm.12310
[6] T. Nissinen, J. Degerman, M. Räsänen, A. Poikonen, S. Koskinen, E. Mervaala, et al. Systemic blockade of acvr2b ligands prevents chemotherapy-induced muscle wasting by restoring muscle protein synthesis without affecting oxidative capacity or atrogenes, Scientific Reports, vol. 6, no. 1, 2016. https://doi.org/10.1038/srep32695
[7] D. DiGirolamo, V. Singhal, X. Chang, S. Lee, & E. Germain‐Lee. Administration of soluble activin receptor 2b increases bone and muscle mass in a mouse model of osteogenesis imperfecta, Bone Research, vol. 3, no. 1, 2015. https://doi.org/10.1038/boneres.2014.42
[8] Y. Lee, A. McPherron, S. Choe, Y. Sakai, R. Chandraratna, S. Lee, et al. Growth differentiation factor 11 signaling controls retinoic acid activity for axial vertebral development, Developmental Biology, vol. 347, no. 1, p. 195-203, 2010. https://doi.org/10.1016/j.ydbio.2010.08.022
[9] S. Lee, L. Reed, M. Davies, S. Girgenrath, M. Goad, K. Tomkinson, et al. Regulation of muscle growth by multiple ligands signaling through activin type ii receptors, Proceedings of the National Academy of Sciences, vol. 102, no. 50, p. 18117-18122, 2005. https://doi.org/10.1073/pnas.0505996102