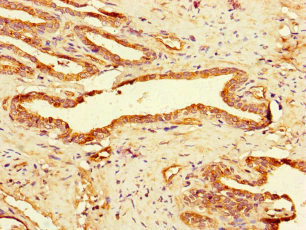
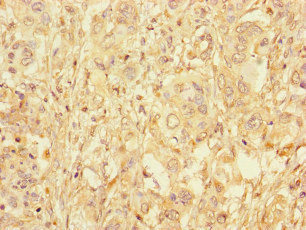
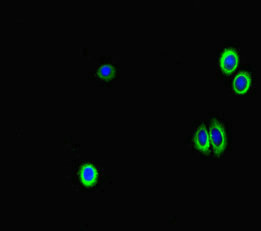
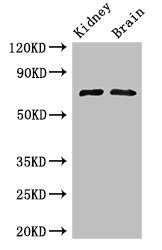
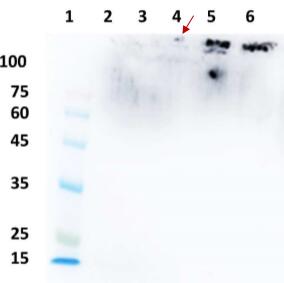
The antibody GAA is generated in rabbits by immunization with a peptide spanning amino acids 601-952 of the recombinant human lysosomal alpha-glucosidase protein. This antibody exists as an unconjugated IgG and can bind to both human and mouse samples. Lysosomal alpha-glucosidase, the target protein of this GAA antibody, is an enzyme that plays a critical role in the lysosomal glycogen breakdown. Besides its function in glycogen metabolism, lysosomal alpha-glucosidase is also involved in autophagy regulation.
The GAA antibody is highly pure, with a protein G purification level of over 95%. It has been extensively tested and validated for use in ELISA, WB, IHC, and IF assays, enabling the detection and localization of lysosomal alpha-glucosidase.