Thanks for your inquiry. For the third one, the accession number and the name you provided are not corresponding. The accession number you provided corresponds to mouse Calmodulin.
Here is the Uniprot link: http://www.uniprot.org/uniprot/?query=NP_033920&sort=score
Considering that you are inquiring for human species, here we provide the details of human HSP90 B1.
Recombinant Human Heat shock protein HSP 90-beta(HSP90AB1)
CSB-YP010808HU >> Yeast
CSB-EP010808HU >> E.coli
CSB-BP010808HU >> Baculovirus
CSB-MP010808HU >> Mammalian cell
Expression Region: 2-724aa, Full Length of Mature Protein
Tag Info: YP: N-terminal 6xHis-tagged; EP, BP, MP: N-terminal 10xHis-tagged and C-terminal Myc-tagged.
Expression Sequence:
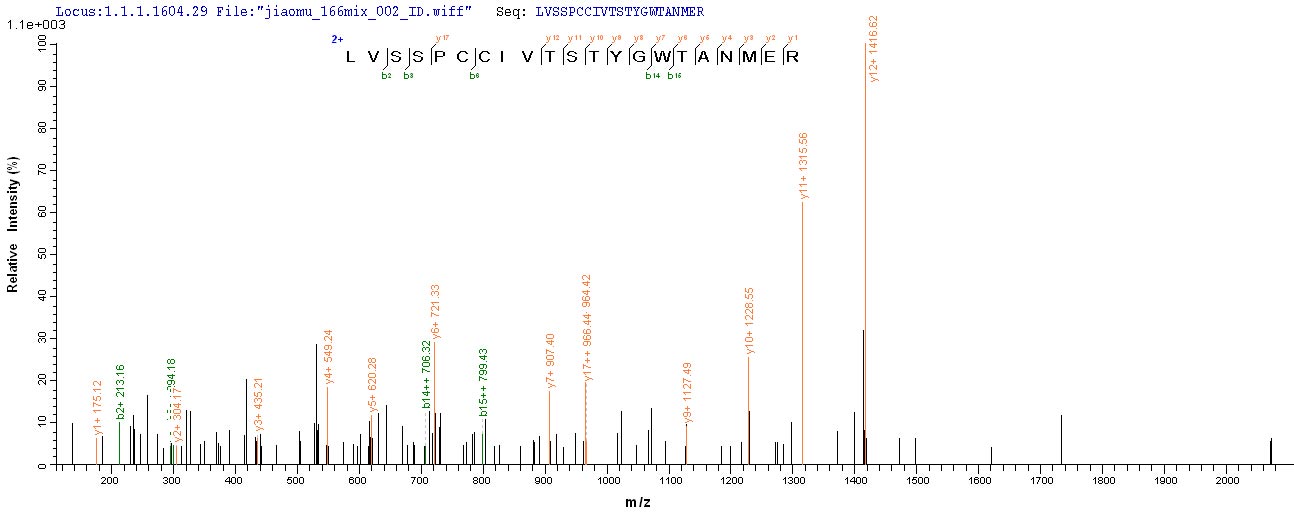
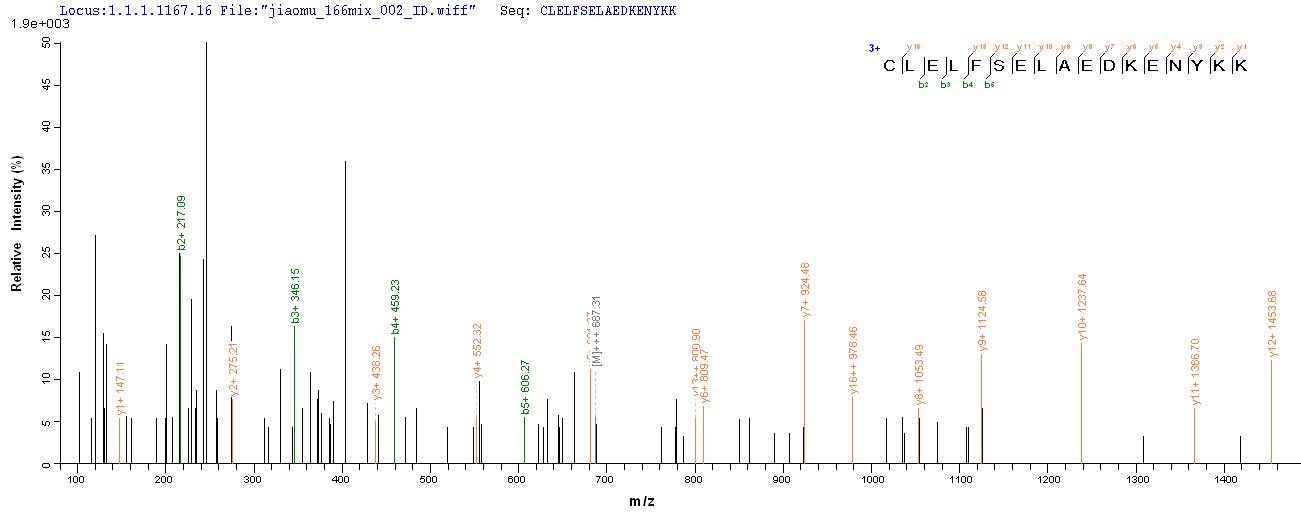
PEEVHHGEEEVETFAFQAEIAQLMSLIINTFYSNKEIFLRELISNASDALDKIRYESLTDPSKLDSGKELKIDIIPNPQERTLTLVDTGIGMTKADLINNLGTIAKSGTKAFMEALQAGADISMIGQFGVGFYSAYLVAEKVVVITKHNDDEQYAWESSAGGSFTVRADHGPIGRGTKVILHLKEDQTEYLEERRVKEVVKKHSQFIGYPITLYLEKEREKEISDDEAEEEKGEKEEEDKDDEEKPKIEDVGSDEEDDSGKDKKKKTKKIKEKYIDQEELNKTKPIWTRNPDDITQEEYGEFYKSLTNDWEDHLAVKHFSVEGQLEFRALLFIPRRAPFDLFENKKKKNNIKLYVRRVFIMDSCDELIPEYLNFIRGVVDSEDLPLNISREMLQQSKILKVIRKNIVKKCLELFSELAEDKENYKKFYEAFSKNLKLGIHEDSTNRRRLSELLRYHTSQSGDEMTSLSEYVSRMKETQKSIYYITGESKEQVANSAFVERVRKRGFEVVYMTEPIDEYCVQQLKEFDGKSLVSVTKEGLELPEDEEEKKKMEESKAKFENLCKLMKEILDKKVEKVTISNRLVSSPCCIVTSTYGWTANMERIMKAQALRDNSTMGYMMAKKHLEINPDHPIVETLRQKAEADKNDKAVKDLVVLLFETALLSSGFSLEDPQTHSNRIYRMIKLGLGIDEDEVAAEEPNAAVPDEIPPLEGDEDASRMEEVD