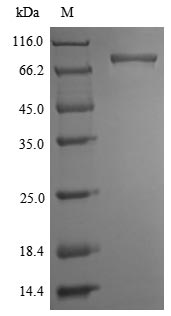
To prepare this Recombinant Mouse Ripk1 protein, the recombinant DNA was required, which was generated by fusing the Ripk1 gene with N-terminal 6xHis-SUMOSTAR tag sequence. Once the recombinant DNA was amplified and purified, a protein expression system, Yeast, was needed for this Ripk1 protein production. After purification, a premium Ripk1 recombinant proteinwas obtained. According to SDS-PAGE, its purity turns out to be 90%+.
Ripk1 (also known as Rinp or Rip) is a protein encoding gene that provides an instruction in making a protein named receptor-interacting serine/threonine-protein kinase 1. The protein encoded by this gene is also commonly called cell death protein RIP1 or receptor-interacting protein 1 (RIP-1), which is highly expressed in gallbladder cancer, and is very important to promoting tumor proliferation and invasion. Current understanding is that RIPK1 can lead to two distinct forms of cell death, including RIPK3‐mediated necroptosis or caspase 8 (Casp8)‐mediated apoptosis.