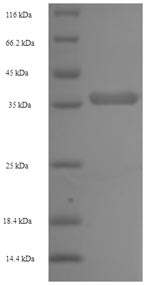
Recombinant Pan troglodytes Lymphotoxin-beta (LTB) is produced using an E. coli expression system, covering the 49-244 amino acid region of the extracellular domain. The protein carries an N-terminal 6xHis-SUMO tag to simplify purification and analysis. SDS-PAGE confirms the product shows greater than 90% purity, which appears suitable for various research applications. This recombinant protein is designed for research use only and is not intended for therapeutic or diagnostic purposes.
Lymphotoxin-beta (LTB) plays a central role in the immune system, mainly through signaling pathways that control lymphoid tissue development and organization. It belongs to the tumor necrosis factor superfamily and seems crucial for maintaining the structure of secondary lymphoid organs. LTB's involvement in immune response regulation has made it an important target in immunology research, especially in studies exploring lymphoid organogenesis and immune system development.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Pan troglodytes Lymphotoxin-beta (LTB) is a cytokine that requires precise folding, proper trimerization with its partner LTA (Lymphotoxin-alpha), and specific tertiary structure for its functional activity in TNF receptor signaling. The E. coli expression system cannot provide the eukaryotic folding environment necessary for correct trimerization and lacks the required partner protein LTA. The N-terminal 6xHis-SUMO tag (~15 kDa), while not larger than the LTB extracellular domain itself (~22 kDa), is still substantial and may sterically interfere with the protein's critical trimerization interfaces and receptor-binding domains. The probability of correct folding leading to a functional, trimerization-competent form is extremely low.
1. Antibody Development and Cross-Reactivity Testing
This application has severe limitations. While antibodies can be generated against linear epitopes, the immune response will be heavily biased towards the large, foreign SUMO tag. Consequently, the antibodies are unlikely to recognize conformational epitopes on the native, trimerized LTB-LTA complex in a physiological context.
2. Protein Stability and Folding Studies
Basic biophysical characterization can be performed, but the results will not reflect the stability of the native trimer. The properties measured (e.g., thermal stability) will be those of a monomeric, tag-fusion protein. The SUMO tag will dominate the biophysical behavior, providing no meaningful insight into the stability of the functional cytokine complex.
Final Recommendation & Action Plan
This SUMO-tagged LTB fragment expressed in E. coli is unsuitable for studies aiming to understand the native biology of Lymphotoxin-beta. The fundamental limitation is its inability to form the mandatory heterotrimeric complex with LTA, a requirement for all functional activity. Comparative and interaction studies should be avoided entirely as they will produce biologically misleading data. Applications 1 and 2 have severe, inherent limitations and will not yield insights relevant to the functional protein. For reliable LTB research, it is essential to use a system that co-expresses LTB with LTA (e.g., in mammalian cells) to produce the correct, trimerized complex.