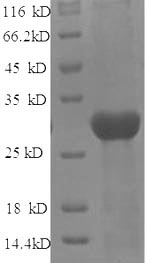
Recombinant Rat Protein-lysine 6-oxidase (Lox) is produced in E. coli and spans the full length of the mature protein from amino acids 163 to 411. This high-purity preparation exceeds 90% purity as confirmed by SDS-PAGE and includes an N-terminal 6xHis-tag for easier purification and detection. The recombinant protein is designed strictly for research applications and appears suitable for various experimental approaches.
Protein-lysine 6-oxidase (Lox) serves as a key enzyme for cross-linking collagen and elastin, which means it plays a significant role in maintaining structural integrity of the extracellular matrix. The enzyme's activity seems essential during connective tissue remodeling and has become a central focus in studies examining tissue development and repair. Many researchers explore Lox within the context of fibrosis and cancer metastasis, given its apparent involvement in these critical biological pathways.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Rat Lox is a copper-dependent enzyme that requires precise folding, proper copper ion coordination, and specific post-translational modifications (including lysine-derived quinone cofactor formation) for its enzymatic activity in collagen cross-linking. The E. coli expression system cannot provide the necessary copper incorporation machinery or the complex post-translational modifications required for functional Lox activity. The N-terminal 6xHis-tag may sterically interfere with the protein's active site or copper-binding domains. While the mature protein region (163-411aa) contains the catalytic core, the probability of correct folding with functional enzymatic activity is extremely low without copper supplementation and proper cofactor formation.
This protein can be used as an immunogen to generate antibodies, as antibody development relies on antigenic sequence recognition rather than functional enzymatic activity. The mature protein region provides specific epitopes for generating antibodies against rat Lox. The high purity (>90%) ensures minimal contamination-related issues during immunization. This E. coli-expressed Lox is unsuitable for functional enzymatic studies due to the essential requirements for copper incorporation and post-translational cofactor formation that cannot be met in this expression system. For reliable Lox research requiring enzymatic activity, use a mammalian-expressed protein with proper copper loading and cofactor formation, or supplement with copper and oxidizing conditions during purification with extensive functional validation.