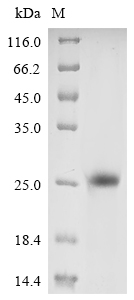
Recombinant Vaccinia virus Protein L1 (L1R) is produced in a yeast expression system and includes a partial sequence from amino acids 2 to 183. The protein carries an N-terminal 10xHis tag for easier purification and detection. SDS-PAGE analysis confirms the product achieves greater than 90% purity, which should meet the standards needed for reliable experimental work.
The L1 protein appears to play a crucial role in the Vaccinia virus life cycle, particularly during viral membrane assembly and maturation. Being part of the poxvirus family, L1 represents an important target for researchers studying viral entry and how viruses interact with their hosts. Its structural and functional features make it a valuable tool for investigating viral pathogenesis and vaccine development, though the complexity of viral systems means other factors likely contribute as well.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
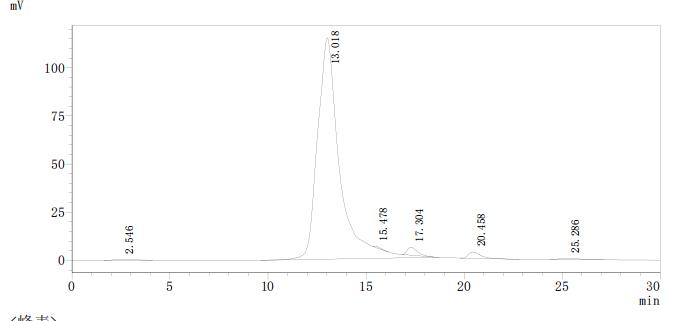
Based on the provided information, the folding state and bioactivity of this recombinant L1R protein fragment are unknown and cannot be assumed. While the high purity (>95% by SEC-HPLC) indicates a monodisperse preparation suggestive of a homogeneous sample, the protein is expressed as a partial fragment (2-183aa) in a yeast system. Vaccinia virus L1R is a membrane protein that requires correct disulfide bond formation for its function in viral entry. Although yeast can perform eukaryotic post-translational modifications, the N-terminal His-tag and the truncation may affect the native structure. The protein's ability to bind its physiological targets (e.g., cell surface receptors or other viral proteins) remains unverified. Therefore, applications relying on specific biological interactions are speculative without validation.
1. Antibody Development and Immunoassay Research
This recombinant L1R fragment is suitable for use as an immunogen to generate antibodies. The high purity and His-tag facilitate purification and immobilization for screening. However, the antibodies generated will be against this specific fragment. Their ability to recognize the full-length, natively folded L1 protein in the context of the mature virion (MV) or intracellular mature virion (IMV) is not guaranteed and must be empirically validated. The recombinant L1R protein is reliable for developing ELISA assays to detect antibodies against this immunogen.
2. Protein-Protein Interaction Studies
The His-tagged protein can be used in pull-down assays. However, the utility for identifying physiologically relevant interactions is contingent on correct folding. L1R's role in viral entry involves specific interactions; if this fragment is misfolded, it may not bind authentic partners, leading to false negatives or non-specific bindings. This application is high-risk without prior confirmation of native folding and activity.
3. Biochemical Characterization and Binding Assays
This recombinant L1R protein is well-suited for biochemical characterization of its intrinsic properties, such as thermal stability and pH tolerance. However, "binding assays" to measure interactions with "potential ligands or other viral proteins" should not be attempted until the recombinant L1R protein's own bioactivity is confirmed. Without validation, binding data would be uninterpretable. The application should focus on stability and biophysical profiling first.
4. Vaccine Research and Immunogenicity Studies
This recombinant L1R protein can be evaluated as a subunit vaccine candidate. The key advantage is that antibody responses often target linear epitopes, so correct folding may be less critical for immunogenicity than for function. However, the protective efficacy of any immune response generated will depend on whether the antibodies can recognize the native protein on the virus. Preclinical studies can proceed, but the correlation between immunogenicity and protection must be established experimentally.
Final Recommendation & Action Plan
The immediate priority is to validate the recombinant L1R protein's bioactivity using a functional assay, such as its ability to bind known targets (e.g., specific antibodies or receptors) or to elicit neutralizing antibodies in a viral entry inhibition assay. Given the high SEC-HPLC purity, the protein is an excellent candidate for such validation. If functional activity is confirmed, it becomes highly valuable for interaction studies (Application 2) and binding assays (Application 3). If inactive, its use should be restricted to antibody generation (Application 1) and vaccine immunogenicity studies (Application 4), with the clear understanding that the immune response may not fully reflect immunity to the native viral protein. Biochemical characterization (Application 3) remains valid regardless of activity. All applications should include controls to account for potential tag-mediated interactions.