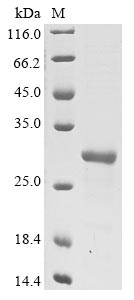
This recombinant Vaccinia virus Protein L1 (L1R) is expressed in E. coli and corresponds to amino acids 2-183 of the L1R protein. The construct includes both an N-terminal 10xHis-tag and a C-terminal Myc-tag, which should make purification and detection more straightforward. SDS-PAGE analysis confirms the protein achieves greater than 85% purity - a level that appears suitable for most research purposes.
Protein L1 represents a crucial component of the Vaccinia virus envelope. Its role in the viral life cycle seems particularly important for virus assembly and egress, though the exact mechanisms may be more complex than initially understood. This makes L1 an intriguing target for researchers trying to piece together viral replication patterns and pathogenesis. Scientists often turn to L1 when investigating how viral infections progress and where therapeutic interventions might prove effective.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant Vaccinia virus L1R is expressed in E. coli, a prokaryotic system that is generally unsuitable for producing functional eukaryotic viral membrane proteins. L1R is a viral membrane protein that requires precise folding, proper disulfide bond formation, and likely specific post-translational modifications for its role in viral entry and membrane fusion. The protein is expressed as a partial fragment (2-183aa) with dual tags (N-terminal 10xHis and C-terminal Myc), which may significantly interfere with proper folding and membrane association. E. coli lacks the eukaryotic chaperones and modification machinery necessary for the correct folding of complex viral membrane proteins. Since activity is unverified, the protein cannot be assumed to be correctly folded or bioactive without experimental validation of its structural integrity and membrane-binding capability.
1. Antibody Development and Immunoassay Studies
The recombinant L1R fragment can serve as an effective immunogen to generate antibodies that recognize linear epitopes within the 2-183aa region, even when the protein is misfolded. The dual tags facilitate purification and detection. However, antibodies may not recognize conformational or modification-dependent epitopes of native, membrane-associated L1R in viral particles. Validation against native L1R from vaccinia virus is recommended.
2. Protein-Protein Interaction Studies
This application is high-risk without folding validation. While the tags enable technical feasibility for pull-down assays, if L1R is misfolded (as expected in E. coli), it will not interact physiologically with true binding partners. Viral membrane proteins require specific conformations for proper interactions with host cell receptors and other viral components. Identified interactions could be non-physiological artifacts. This application should not be pursued without confirmation of proper folding.
3. ELISA-Based Binding Assays
This application should be approached with caution. If L1R is misfolded, binding assays will not reflect biological reality. The tags enable technical development of ELISA formats, but results may be misleading without proper folding validation. Membrane protein binding studies require native conformation for meaningful results.
4. Western Blot Standard and Positive Control
This application is appropriate and represents a safe use case. The recombinant L1R protein can serve as a positive control for Western blot detection of L1R, providing a known molecular weight reference. The dual tags offer multiple detection options. However, the migration pattern may not exactly match native L1R due to potential differences in folding and modification.
Final Recommendation & Action Plan
Given the high probability of misfolding in E. coli for this viral membrane protein, we recommend first performing biophysical characterization to assess folding quality. This should include circular dichroism spectroscopy for secondary structure analysis and non-reducing SDS-PAGE for disulfide bond assessment. Antibody development and use as a Western blot standard can proceed as the safest applications. Avoid all functional studies (interactions, binding assays) until proper folding is validated. For reliable L1R functional studies, obtain full-length protein from eukaryotic expression systems capable of proper membrane protein folding and post-translational modifications. Always validate findings with native L1R from vaccinia virus particles when possible.