T cells are crucial for the immune system, acting as the body’s defense against pathogens and tumors. Understanding the intricate process of T cell differentiation is essential for unraveling how these immune warriors develop and function. From their origins in the bone marrow to their maturation in the thymus and subsequent activation in peripheral tissues, each stage of T cell development is characterized by specific changes in surface markers and functional capabilities.
In this article, we will explore the key stages of T cell differentiation, highlighting the factors that regulate T cell differentiation, metabolic pathways and signaling pathways that modulate T cell differentiation, as well as critical surface markers that define their evolution and the implications for immunological health and disease.
Table of Contents
1. What Is T Cell Differentiation?
T cell differentiation is a highly regulated process in which hematopoietic stem cells (HSC) in the bone marrow develop into common lymphoid progenitor (CLP) cells, enter the thymus for maturation, and expand into specialized subsets that perform specific functions.
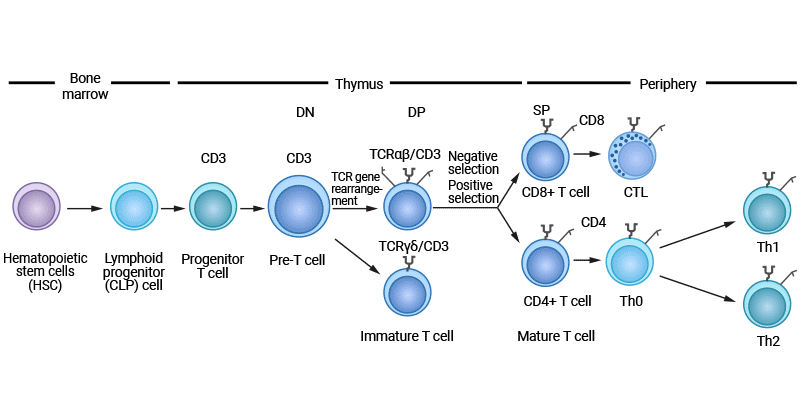
Figure 1. T cell development
T cell differentiation is important role in establishing effective immune defense mechanisms, maintaining immune tolerance, and fighting diseases such as cancer.
2. How Do T Cells Differentiate?
The thymus is the primary place of T cell differentiation and development, where lymphoid progenitor cells undergo a series of developmental steps leading to the formation of mature T cells capable of responding to antigens.
2.1 Stem Cell Stage
T cell differentiation begins with hematopoietic stem cells in the bone marrow. Hematopoietic stem cells develop into common lymphoid progenitor cells, which subsequently migrate to the thymus.
2.2 Development in the Thymus
The thymus is the primary site of T cell differentiation and development, where lymphoid progenitor cells from the bone marrow migrate to undergo a series of developmental stages including double negative (DN), double positive (DP), and single positive (SP).
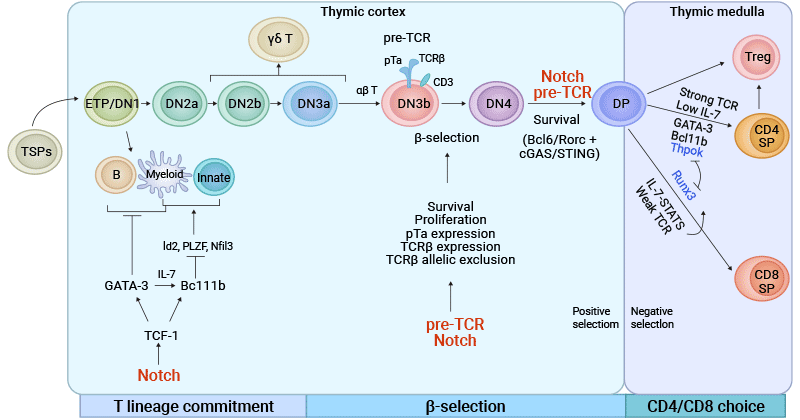
Figure 2. Development and Regulatory Mechanism of T cells in the Thymus
The picture is cited from: https://www.nature.com/articles/s41392-023-01471-y
2.2.1 Double-Negative (DN) Stage
In the DN stage, lymphoid progenitor cells that migrate from the bone marrow to the thymic cortex specialize into progenitor T (pro-T) cells, which further differentiate into pre-T cells. This process is known as T cell lineage commitment, launched by the Notch signaling pathway [1,2].
Pre-T cells do not yet express CD4 and CD8 coreceptors, so they are called double-negative (CD4-CD8-). These cells begin to rearrange the genes encoding the T cell receptor (TCR) β chain. After TCRβ rearrangement is completed, pre-TCR expressing DN cells assembled with the TCRβ chain as well as pTα and CD3 molecules (called β-selection) differentiate into αβ T cells, otherwise, they tend to become γδ T cells [3-5].
2.2.2 Double-Positive (DP) Stage
Once they have successfully rearranged the TCRβ-chain genes and expressed the pre-TCR, DN cells enter the DP stage, in which they express both CD4 and CD8 co-receptors. Notch signaling and formation of pre-TCR have been shown to promote DN to DP stage transition.
DP T cells undergo positive selection in the thymus cortex. Positive selection is aimed at whether TCRs can effectively recognize autoantigens presented by thymus epithelial cells. This process ensures that only T cells that are able to bind to their own MHC molecules survive and continue to develop.
2.2.3 Single-Positive (SP) Stage
Following positive selection, DP cells carrying MHC class I- or MHC class II-TCRs differentiate into either CD8+ or CD4+ T cells, known as CD4/CD8 lineage choice. This stage, called DP stage, occurs in the thymic medulla [6,7].
The high affinity binding SP cells (i.e., autoreactive T cells) undergo apoptosis, and a few of them differentiate into regulatory T cells (Tregs). SP cells that fail to bind survive to become mature T cells and enter the peripheral immune organs.
2.3 T Cells Migration out of the Thymus and Development in Peripheral Lymphoid Organs
T cells that have undergone positive and negative selection eventually develop into CD4+ helper T cells and CD8+ cytotoxic T cells, which migrate out of the thymus through the skin-medullary junction and enter peripheral lymphoid organs such as lymph nodes and spleen. In the periphery, these T cells are quiescent, waiting for antigen stimulation.
T cell activation is usually initiated by signals provided by antigen-presenting cells (APCs). When APCs present the antigen through major histocompatibility complex (MHC) molecules, TCR binds to the antigen-MHC complex, initiating the activation of T cells. CD4+ T cells recognize antigens on MHC Class II molecules, while CD8+ T cells recognize antigens on MHC Class I molecules.
Antigen recognition alone is not enough to fully activate T cells. Co-stimulatory signals are crucial and are usually achieved by binding of co-stimulatory molecules such as CD28 on the surface of T cells to ligands such as B7 on the surface of APCs. This process enhances T cell activation.
During activation, APCs secrete various cytokines such as IL-2, which further promote the proliferation and differentiation of T cells.
2.5 Proliferation and Differentiation
Once activated, T cells rapidly proliferate to form a large number of cloned cells, which have the same TCR and can specifically recognize the same antigen. Depending on the received signals and environmental factors, T cells differentiate into different effector cell types.
CD8+ T cells become cytotoxic T lymphocytes (CTLs) that recognize and kill infected or mutated cells, while CD4+ T cells differentiate into multiple subsets such as Th1, Th2, Th17, etc. Th1 cells are mainly involved in cellular immune responses and can activate macrophages and promote the activity of CD8+ T cells. Th2 cells mainly participate in humoral immune responses, promoting B cells to produce antibodies. Th17 cells are associated with autoimmunity and inflammatory responses. Tregs primarily prevent the immune system from attacking its own tissues and maintain immune tolerance.
After the response ends, some effector T cells transform into memory T cells, which can respond quickly when they encounter the same antigen again.
3. What Factors Regulate T Cell Differentiation?
T cell differentiation is a highly regulated process, which is influenced by many factors, including antigen type and does, cytokines, transcription factors, and immune microenvironment.
Antigen Type and Dose: Different types of antigens have different effects on T cell activation and differentiation. For example, protein antigens are generally more effective in activating T cells, while non-protein antigens may need to work with other molecules to produce an immune response. Low doses of antigen usually lead to T cell tolerance, while high doses of antigen may promote T cell activation and differentiation.
Cytokines: Cytokines play a central role in the activation and differentiation of T cells. Specific cytokines determine T cell lineage.
For instance, IL-2, IL-12, and IFN-γ help CD4+ T cells differentiate into Th1 cells, thereby enhancing cellular immune responses. IL-4 promotes the differentiation of CD4+ T cells into Th2 cells, enhancing immune responses to allergic reactions and parasitic infections. IL-6 and TGF-β can promote the differentiation of Th17 cells, which are important in autoimmune diseases and inflammation.
IL-10 not only promotes the differentiation of Tr1 cells but also inhibits the activity of Th1 and Th2 cells, thus maintaining immune balance and preventing excessive immune responses. IL-27 plays a key role in the development and function of Tregs and promotes the production of Th1 cells.
Transcription factors: Specific transcription factors regulate the expression of genes necessary for each T cell subtype [8]. For example, Foxp3 regulates the differentiation and function of Treg cells [9]. Changes in its expression level directly affect the formation and function of Treg cells. RORγt is a master regulator of Th17 cell differentiation.
TCF1 is a key transcription factor, especially in the early thymic progenitor (ETP) stage, where the expression of TCF1 is essential for the selection of T cell lineage [10]. TCF1 is activated through the Notch signaling pathway, thereby regulating a series of T cell-specific transcription factors such as GATA3 and promoting the development of T cells. TCF1 plays an important role in all stages of T cell development, including the transition from the DN stage to mature T cells.
GATA3 controls T cell development until the DN2 stage by regulating transcription factors associated with T cell identity. The interaction between GATA3 and other transcription factors such as T-bet determines the differentiation of CD4+ T cells into Th1 or Th2 phenotypes. The presence of GATA3 promotes the formation of the Th2 phenotype, while T-bet promotes the development of the Th1 phenotype.
HES1 is one of the downstream effectors of the Notch signaling pathway and usually plays a role in the early stages of T cell development. Hes1 helps maintain the undifferentiated state of T cell precursors by inhibiting premature differentiation.
In pro-T cells, E proteins, including E2A and E2-2 family members, modulate stage-specific expression of Notch and essential T cell lineage genes, regulate Notch-induced expression of GATA3, and finally suppress the development of ILCs in the thymus to establish T cell identity.
Immune Microenvironment: Different tissue environments and pathological states such as inflammation and tumors can affect the behavior and differentiation of T cells. The tumor microenvironment may inhibit the function of T cells through specific cytokines and signaling pathways, leading to tumor immune escape.
4. Metabolic Regulation of T Cell Differentiation
T cell differentiation metabolism is a complex process involving the interaction of multiple metabolic pathways and signaling pathways. At each stage, the metabolic state of T cells affects their function and fate. Distinct T cell subsets exhibit unique metabolic profiles that are specifically adapted to satisfy the bioenergetic requirements at each stage.
The Notch signaling pathway and the IL-7 signaling pathway are the main regulatory factors during the development of T cells in the thymus, including the DN, DP, and SP stages. The Notch signaling pathway promotes the upregulation of aerobic glycolysis, providing energy support for the proliferation and differentiation of T cells [11]. The IL-7 signaling pathway increases the metabolic activity of glycolysis and fatty acid oxidation through the JAK-STAT pathway, supporting cell growth and differentiation [11].
At the SP stage, mature T cells need to switch between glycolysis and fatty acid oxidation to ensure a balance between energy supply and metabolic demand [12]. At this time, cells become more sensitive to nutrients in the environment and can adjust metabolic pathways as needed.
Naive T cells rely on mitochondrial pathways that require minimal nutrient intake, such as the tricarboxylic acid cycle (TCA) and oxidative phosphorylation (OXPHOS).
During T cell activation, cells undergo a transition from a resting state to an activated state, accompanied by a significant change in metabolic patterns, especially a shift toward anabolic metabolism. The binding of the TCR to the antigen-MHC complex initiates a series of downstream signaling processes that ultimately guide cells into a state of proliferation and differentiation [13].
Activation of CD28 can enhance metabolic signals, particularly by activating the mTOR signaling pathway, further promoting glycolysis and cell proliferation [14]. Activated T cells significantly increase the uptake of nutrients, including glucose and amino acids, to meet the needs of rapid proliferation and differentiation. c-Myc is important for T cell activation. It promotes glycolysis and regulates gene expression in other metabolic pathways to support rapid cell proliferation [15].
Once activated, effector T cells undergo metabolic reprogramming, including a shift from mitochondrial responses to glycolysis and glutaminolysis, which are pathways that absorb more nutrients from the surrounding microenvironment.
In the effector T cell stage, cells meet the needs of their rapid proliferation and effector function through glycolysis and increased mitochondrial oxidative phosphorylation. The metabolism of T cells is regulated by various cytokines such as IL-2 to promote their proliferation and differentiation [13,15].
In regulatory T cells, FOXP3 regulates metabolism by inhibiting Myc and glycolysis, enhancing OXPHOS, and increasing nicotinamide adenine dinucleotide (NAD) oxidation [16].
Memory T cells typically exhibit higher levels of fatty acid oxidation and lower glycolytic activity, which enables them to maintain activity in a long-term dormant state. At this time, the regulation of metabolic pathways mainly depends on specific signal transduction pathways such as the PI3K-AKT-mTOR signaling pathway, which is closely related to cell growth and metabolism [17].
5. T Cell Differentiation Markers
T cell differentiation markers are an important research area in immunology, and they play a key role in the development and function of T cells. T cells undergo a series of differentiation processes in the thymus, which involves changes in the expression of multiple markers, reflecting their different developmental stages and functional states.
| T Cell Differentiation Stages |
Markers |
| NaiveT cell |
CD62L, CD197 (CCR7), CD45RA, CD27, CD28 |
| Killer CD8+T cell |
CD8, CD25 (IL-2Rα), KLRG1 |
| Helper CD4+T cell |
CD4, CD183 (CXCR3), CD194 (CCR4) |
| Th1 cell |
CD4, CCR1, CCR5, CXCR3, CD119 (IFNGR1), IL-18Rα, IL-27Rα |
| Th2 cell |
CD4, CCR3 (CD193), CCR4, CCR8 (CD198), CXCR4 (CD184), IL-17RB |
| Th9 cell |
CD4, IL-4Rα, IL-17RB |
| Th17 cell |
CD4, CCR4, CCR6, IL-1RI, IL-6Rα, IL-21R, IL-23R |
| Th22 cell |
CD4, CCR4, CCR6, CCR10, IL-6Rα |
| Treg |
CD4, CD25, CD127 (IL-17RA), CD152,CCR4, FoxP3 |
| Central memory T cells (Tcm) |
CD45RO, CCR7 (CD197), CD25, CD27, CD28 |
| Effector memory T cells (Tem) |
CD45RO, CD57, CD127, KLRG1 |
6. Dysregulated T Cell Differentiation and Diseases
The T cell differentiation is vital for eliminating both intracellular and extracellular pathogens. However, if this process becomes dysregulated, it may lead to a variety of diseases, especially autoimmune and inflammatory diseases.
When human T cell differentiation is abnormal, it may lead to insufficient or incomplete functions of certain types of T cells such as CD4+ and CD8+ T cells. This situation makes it difficult for individuals to fight common pathogens and makes them more susceptible to opportunistic infections, such as those caused by bacteria, fungi, or viruses.
Autoimmune diseases are diseases caused by the immune system mistakenly attacking its own tissues. T cells play a key role in this process, especially in the case of dysregulated differentiation of Tfh cells. Systemic lupus erythematosus (SLE) is an autoimmune disease that affects multiple organs and is often accompanied by abnormal T cell function, resulting in increased reactivity to self-antigens.
In rheumatoid arthritis (RA), dysregulated activation and differentiation of T cells lead to chronic inflammation and damage of the joints. Multiple sclerosis (MS) is an autoimmune disease of the central nervous system in which T cells attack the myelin sheath of nerves, resulting in neurological dysfunction.
T cells also play an important role in allergic reactions. Allergic diseases often involve abnormal immune responses to harmless substances. In allergic rhinitis, differentiation and activation of T cells lead to an overreaction to allergens in the environment. Specific types of T cells such as TH2 cells play an important role in the pathogenesis of asthma, leading to inflammation and hyperreactivity of the airways.
Conclusion
The T cell differentiation and development is a process of continuous migration and self-selection. In this process, T cells continuously "enrich" themselves, acquire the ability of self-recognition and "inclusion", and gradually develop and mature into subgroups with different biological functions, including helper T cells, regulatory T cells, and cytotoxic T cells.
T cell differentiation is highly regulated by metabolic pathways, signaling networks, and transcriptional controls. The interplay of glycolysis, mitochondrial function, and specific transcription factors is essential for the proper differentiation and function of T cells, ultimately shaping the immune response.
References
[1] Wilson, A., MacDonald, H. R. & Radtke, F. Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus [J]. J. Exp. Med. 194, 1003–1012 (2001).
[2] Han, H. et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision [J]. Int. Immunol. 14, 637–645 (2002).
[3] Dutta, A., Zhao, B. & Love, P. E. New insights into TCR beta-selection [J]. Trends Immunol. 42, 735–750 (2021).
[4] Sahni, H. et al. A genome wide transcriptional model of the complex response to pre-TCR signalling during thymocyte differentiation [J]. Oncotarget 6, 28646–28660 (2015).
[5] Harker, N. et al. Pre-TCR signaling and CD8 gene bivalent chromatin resolution during thymocyte development [J]. J. Immunol. 186, 6368–6377 (2011).
[6] Teh, H. S. et al. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells [J]. Nature 335, 229–233 (1988).
[7] Taniuchi, I. CD4 helper and CD8 cytotoxic T cell differentiation [J]. Annu. Rev. Immunol. 36, 579–601 (2018).
[8] Hosokawa H, Rothenberg EV. How transcription factors drive choice of the T cell fate [J]. Nat Rev Immunol. 2021 Mar;21(3):162-176.
[9] Ono M. Control of regulatory T-cell differentiation and function by T-cell receptor signalling and Foxp3 transcription factor complexes [J]. Immunology. 2020 May;160(1):24-37.
[10] Johnson JL, et al. Lineage-Determining Transcription Factor TCF-1 Initiates the Epigenetic Identity of T Cells [J]. Immunity 48, 243–257 e10 (2018).
[11] Zhang, M., Lin, X., et al. (2022) Metabolic regulation of T cell development [J]. Frontiers in Immunology, 13, 946119.
[12] Almeida, L., Lochner, M., Berod, L., & Sparwasser, T. (2016) Metabolic pathways in T cell activation and lineage differentiation [J]. Seminars in Immunology, 28(5), 514-524.
[13] Shyer, J. A., Flavell, R. A., & Bailis, W. (2020) Metabolic signaling in T cells [J]. Cell Research, 30(8), 649-659.
[14] Ananieva, E. A., Powell, J. D., & Hutson, S. M. (2016) Leucine Metabolism in T Cell Activation: MTOR Signaling and Beyond [J]. Advances in Nutrition, 7(4), 798S-805S.
[15] Ma, S., Ming, Y., Wu, J., & Cui, G. (2024) Cellular metabolism regulates the differentiation and function of T-cell subsets [J]. Cellular & Molecular Immunology, 21(5), 419-435.
[16] Kempkes, R. W., Joosten, I., Koenen, H. J., & He, X. (2019) Metabolic Pathways Involved in Regulatory T Cell Functionality [J]. Frontiers in Immunology, 10, 483290.
[17] Kishton, R. J., Sukumar, M., & Restifo, N. P. (2017) Metabolic Regulation of T Cell Longevity and Function in Tumor Immunotherapy [J]. Cell Metabolism, 26(1), 94-109.
CUSABIO team. Immune Activation: Insights into T Cell Differentiation. https://www.cusabio.com/c-21194.html



Comments
Leave a Comment