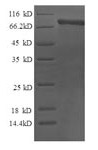
Calpain-2 catalytic subunit (CAPN2) CSB-EP004496HU is a recombinant full Length of mature protein carrying a 6xHis-tag at the N-terminus. This protein containing amino acid 20-700 of human CAPN2 was expressed in E.coli. It migrated to a band of 85-90 kDa on the reducing SDS-PAGE gel. Its purity was measured by up to 90%. The recombinant CAPN2 protein may be used as an immunogen for antibody production or in the studies of calpain-2-related cancers. In-stock CAPN2 proteins are available.
CAPN2, a large catalytic subunit, makes up the m-calpain with a small regulatory subunit CAPNS1. m-Calpain activation limits the extent of long-term potentiation (LTP) and is neurodegenerative. Extrasynaptic NMDAR (N-methyl-D-aspartate receptor) activation induces m-calpain activation, resulting in STEP degradation and subsequent activation of the p38 prodeath pathway, ultimately leading to neurotoxicity.