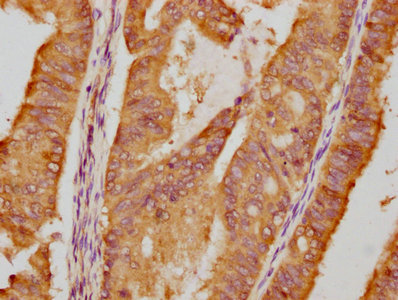
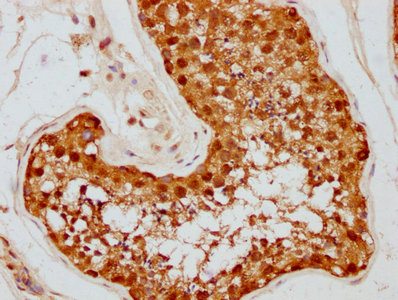
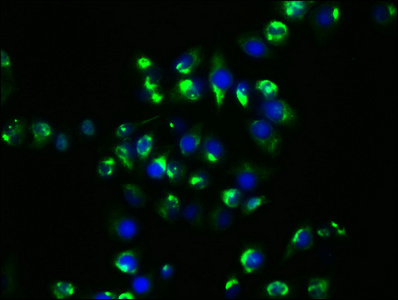
The HSP90AA1 recombinant monoclonal antibody is generated using DNA recombinant technology and in vitro genetic manipulation. Initially, an animal is immunized with a synthesized peptide derived from human HSP90AA1, leading to the isolation and selection of B cells. The positive B cells are screened and subjected to single clone identification. The light and heavy chains of the HSP90AA1 antibody are then amplified through PCR and inserted into a plasmid vector, creating a recombinant vector. This vector is introduced into a host cell line for antibody expression. The HSP90AA1 recombinant monoclonal antibody is purified from the cell culture supernatant using affinity chromatography. This antibody exhibits specific binding to human HSP90AA1 protein and is recommended for use in ELISA, IHC, and IF applications.
The HSP90AA1 protein mainly acts as a chaperone protein that helps other proteins fold correctly and maintain their functional conformation. HSP90AA1 is involved in multiple cellular processes, including signal transduction, protein degradation, DNA repair, and cell cycle control. In addition to its role as a chaperone, HSP90AA1 is also involved in the assembly and activation of several important signaling complexes, including steroid hormone receptors, kinases, and transcription factors. It helps to stabilize these complexes, allowing them to transduce signals more effectively and regulate downstream gene expression.