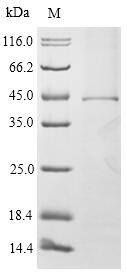
Recombinant Mouse High affinity immunoglobulin epsilon receptor subunit alpha (Fcer1a) is produced using an in vitro E.coli expression system, spanning the full mature protein length from amino acids 24 to 250. This protein carries an N-terminal 10xHis-SUMO tag, which provides easier purification and enhanced stability. The product achieves over 90% purity as confirmed by SDS-PAGE, making it appropriate for various research applications.
The High affinity immunoglobulin epsilon receptor subunit alpha (Fcer1a) appears to be a crucial component of the immunoglobulin E (IgE) receptor complex, largely involved in allergic responses. It likely plays a central role in the signal transduction pathway that triggers mediator release from mast cells and basophils. Understanding Fcer1a may be essential for research into allergy mechanisms and developing therapeutic interventions.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Mouse Fcer1a is an immunoglobulin superfamily receptor that requires precise folding, proper disulfide bond formation (with 7 conserved disulfide bonds), and specific domain organization for its high-affinity IgE binding activity. The in vitro E. coli expression system (cell-free) cannot provide the necessary eukaryotic folding environment, oxidative conditions for disulfide bond formation, or post-translational modifications required for this complex receptor. The large N-terminal 10xHis-SUMO tag (∼15 kDa) may sterically interfere with the protein's functional domains and binding interfaces. While cell-free systems can produce soluble proteins, the probability of correct folding with functional IgE binding activity is extremely low.
1. Antibody Development and Validation Studies
This application is highly suitable as antibody development primarily relies on linear epitope recognition rather than functional protein folding. The protein can generate antibodies against the Fcer1a sequence (24-250aa), though these antibodies may not recognize conformational epitopes dependent on proper disulfide bonding in the native receptor. The high purity (>90%) ensures minimal contamination-related issues during immunization protocols.
2. Biochemical Characterization
Basic biophysical characterization can be performed, but it will not reflect native structure because the expression system cannot support proper disulfide bond formation. Techniques like circular dichroism may detect secondary structure but cannot verify functional disulfide bonding, and structural biology techniques requiring native folding will not yield meaningful results for this misfolded protein.
3. ELISA Development and Immunoassay Applications
This application is suitable for detecting antibodies against linear epitopes but not for functional ligand binding studies because immunoassays for antibody detection rely on sequence recognition rather than functional conformation. The protein can serve as a coating antigen for antibody detection, but cannot model native IgE-receptor interactions due to the lack of proper disulfide bonding.
Final Recommendation & Action Plan
The in vitro E. coli expression system is fundamentally unsuitable for producing a functional Fcer1a receptor due to its inability to form the essential disulfide bonds required for proper immunoglobulin domain folding. This recombinant protein is only suitable for Application 1 (antibody development against linear epitopes) and basic aspects of Application 3 (ELISA for antibody detection). Structural and functional studies are not recommended because they require precise disulfide-dependent tertiary structure and native receptor conformation with correct disulfide bonds, respectively, that cannot be achieved in the cell-free expression system. For reliable Fcer1a research requiring functional activity, use mammalian expression systems that support proper disulfide bond formation and receptor folding.