TNFRSF13C (BAFF-R), a member of the TNF receptor superfamily, is pivotal for immune regulation. This review delves into its background, mechanisms, signaling pathways, associated diseases, and drug development progress.
1. Overview of TNFRSF13C (BAFF-R)
TNFRSF13C, or BAFF-R, regulates B cell development and survival, and is mainly found on mature, activated B cells and plasma cells. It binds to BAFF to regulate B cell development and maintain their survival [1]. Its structure includes an extracellular cysteine-rich domain for ligand binding and receptor multimerization, and an intracellular domain that recruits TRAF family proteins to transmit signals.
Abnormal BAFF-R function can lead to B cell homeostasis disorders. For instance, homozygous mutations can cause B cell developmental arrest, lymphocytopenia, hypogammaglobulinemia, and humoral immune deficiency [2-3]. Additionally, its single nucleotide polymorphisms are associated with immunodeficiency, autoimmune diseases, and tumors.
2. TNFRSF13C (BAFF-R) Related Signaling Pathways
After TNFRSF13C is activated, it primarily functions through the following three signaling pathways:
After TNFRSF13C binds to its ligand BAFF, its intracellular domain interacts with TRAF2 and TRAF3, activating the IκB kinase (IKK) complex. This leads to IκB phosphorylation and degradation, releasing NF-κB to enter the nucleus to regulate gene expression, promoting B cell survival and proliferation, and inhibiting apoptosis. It plays a central role in B cell development and immune responses.
After TNFRSF13C is activated, it interacts with TRAFs to activate upstream kinases, which in turn activate ERK1/2, JNK, and p38 MAPK. ERK1/2 mainly promotes B cell proliferation and differentiation. JNK is involved in cellular stress responses and survival/proliferation regulation. p38 participates in inflammatory responses and cytoskeleton remodeling, affecting B cell migration, positioning, and cell cycle progression.
After TNFRSF13C binds to its ligand, its intracellular domain interacts with TRAFs to recruit the PI3K complex. Once activated, PI3K converts PIP2 into PIP3. PIP3 recruits AKT and PDK1 to the cell membrane. After AKT is phosphorylated and activated, it enhances B cell survival by inhibiting pro-apoptotic protein BAD and promoting anti-apoptotic protein expression. It also promotes the expression of cell cycle-related proteins, driving cell cycle progression.
3. TNFRSF13C (BAFF-R) and Related Diseases
3.1 Immunodeficiency Diseases
● Immunodeficiency Diseases
Some HIGM patients have TNFRSF13C gene mutations. For example, HIGM2 is caused by TNFRSF13C gene mutations. When TNFRSF13C function is impaired, it cannot bind to BAFF and APRIL normally. This leads to impaired CSR and SHM processes in B cells. Patients cannot produce normal levels of IgG, IgA, and IgE, but IgM levels rise. They are prone to recurrent bacterial infections and have a higher incidence of autoimmune diseases.
● Common Variable Immunodeficiency (CVID)
TNFRSF13C homozygous mutations (e.g., p.Pro21Arg, P21R) can cause receptor multimerization defects, reducing BAFF binding and NF-κB signaling. This leads to reduced B cells, low IgG/IgM, normal IgA, and impaired polysaccharide vaccine responses [4]. For instance, patients with P21R and H159Y compound heterozygous mutations exhibit severe humoral immune deficiency and autoimmune enteropathy.
● Congenital Agammaglobulinemia
TNFRSF13C homozygous deletion (e.g., 24bp deletion) causes B cell developmental arrest at the CD10+ transitional stage, with almost no circulating B cells. However, mucosal IgA+ plasma cells can still differentiate [5].
3.2 Autoimmune Diseases
● Systemic Lupus Erythematosus (SLE)
SLE patients show significantly elevated levels of TNFRSF13C and its ligands BAFF and APRIL. Overactivation of TNFRSF13C leads to abnormal B cell activation and proliferation, producing large amounts of autoantibodies (e.g., antinuclear antibodies, anti-double-stranded DNA antibodies). These form immune complexes that deposit in tissues and organs, causing inflammatory damage. Common manifestations include malar rash, arthritis, and lupus nephritis. TNFRSF13C promotes inflammatory factor release through NF-κB and MAPK signaling pathways, exacerbating immune dysregulation [6]. In addition to conventional treatments, biological agents like belimumab inhibit BAFF, indirectly blocking TNFRSF13C-ligand binding, reducing autoantibody production, and improving the condition.
● Rheumatoid Arthritis (RA)
RA patients have elevated TNFRSF13C and BAFF/APRIL levels in synovial tissue and serum. In inflammatory synovial sites, TNFRSF13C activates B cells to produce autoantibodies like rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (ACPA). These participate in synovial inflammation and progression. The autoantibodies activate complement systems and attract inflammatory cells, leading to synovial hyperplasia, pannus formation, and cartilage/bone destruction. TNFRSF13C promotes inflammatory cell infiltration and cytokine production (e.g., IL-1β, IL-6) via NF-κB and MAPK activation. In addition to conventional therapies, biological agents like atacicept are being studied to block BAFF and APRIL binding to TNFRSF13C, inhibiting abnormal B cell activation and alleviating joint inflammation.
● Multiple Sclerosis (MS)
MS patients show abnormal TNFRSF13C expression in the central nervous system (CNS). B cells abnormally aggregate and activate in the CNS via TNFRSF13C binding to BAFF and APRIL. Activated B cells act as antigen-presenting cells, activating T cells and producing autoantibodies like anti-myelin oligodendrocyte glycoprotein (MOG) antibodies. This causes demyelination and axonal damage, leading to symptoms like vision impairment, limb weakness, sensory abnormalities, and ataxia. TNFRSF13C activation promotes inflammation and neurodamage by enabling inflammatory cells to cross the blood-brain barrier. MS treatment mainly involves immunomodulators and immunosuppressants, with TNFRSF13C pathway-targeted therapies (e.g., inhibiting BAFF/APRIL activity) being explored to reduce abnormal B cell aggregation and activation in the CNS.
3.3 Tumor Diseases
● B-Cell Lymphoma
Abnormal TNFRSF13C expression is common in B-cell lymphoma. Binding to BAFF and APRIL, TNFRSF13C activates signaling pathways like NF-κB and MAPK, promoting B-cell lymphoma cell proliferation and survival. It also helps tumor cells evade apoptosis, driving tumor progression. For example, in diffuse large B-cell lymphoma (DLBCL), TNFRSF13C overexpression is linked to poor prognosis. Engineered T cells targeting BAFF-R can specifically kill tumor cells without harming normal hematopoietic stem cells [7].
● Multiple Myeloma
In multiple myeloma, elevated BAFF and APRIL levels in the bone marrow microenvironment bind to TNFRSF13C to activate signaling pathways, promoting abnormal plasma cell proliferation and survival. TNFRSF13C also regulates interactions between tumor cells and bone marrow stromal cells, driving bone destruction and tumor growth. Inhibiting TNFRSF13C pathways may suppress multiple myeloma progression and improve patient outcomes.
● Chronic Lymphocytic Leukemia
Chronic lymphocytic leukemia (CLL) is characterized by abnormal B cell proliferation and accumulation. TNFRSF13C aberrant expression in CLL cells correlates with disease progression and chemoresistance. TNFRSF13C activation promotes CLL cell survival and proliferation via relevant pathways and affects chemosensitivity. TNFRSF13C-targeted therapies may help overcome chemoresistance, enhancing treatment efficacy.
4. Latest Drug Development Progress Related to TNFRSF13C (BAFF-R)
Drugs targeting TNFRSF13C include monoclonal antibodies and CAR-T therapies, for autoimmune and tumor diseases. Several are in advanced clinical stages. Below are some pipelines (as of April 25, 2025):
| Drug |
Target |
Drug Type |
Indications |
Developer |
Phase |
| Ianalumab |
BAFF-R |
Monoclonal Antibody |
Systemic Lupus Erythematosus, Immune Thrombocytopenia, Warm Autoimmune Hemolytic Anemia, Lupus Nephritis |
Novartis Pharmaceuticals Canada, Inc., Beijing Novartis Pharma Co., Ltd., etc. |
Phase 3 |
| ESG-206 |
BAFF-R |
Monoclonal Antibody |
Immune Thrombocytopenia, CD19-Expressing Malignancies, B-Cell Malignancies, Diffuse Large B-Cell Lymphoma |
Shanghai Sci-Gen Biotech Co., Ltd. |
Phase 1/2 |
| Anti-BAFFR CAR-T (Tianjin Medical University General Hospital) |
BAFF-R |
CAR-T |
Neuromyelitis Optica |
Tianjin Medical University General Hospital |
Phase 1/2 |
| APRIL-BAFF-Bicephali CAR-T cells (Yake Biotechnology) |
APRIL x BAFF-R |
CAR-T |
Relapsed/Refractory Multiple Myeloma |
Shanghai Yake Biotechnology Co., Ltd. |
Phase 1/2 |
| Anti-BAFF receptor CAR-T cell therapy (City of Hope National Medical Center/PeproMene Bio) |
BAFF-R |
Autologous CAR-T |
Relapsed/Refractory B-Cell Lymphoma, Recurrent B-Cell Acute Lymphoblastic Leukemia |
City of Hope National Medical Center, PeproMene Bio, Inc. |
Phase 1 |
| RTX-C001 |
BAFF-R |
CAR-T |
Lymphoma |
Renhaim, Inc. |
Phase 1 |
| MC-10029 |
BAFF-R |
CAR-T |
B-Cell Malignancies, Recurrent Diffuse Large B-Cell Lymphoma, Refractory Diffuse Large B-Cell Lymphoma |
Mayo Clinic |
Phase 1 |
| LMY-920 |
BAFF-R x BCMA x TACI |
Autologous CAR-T |
Relapsed/Refractory Chronic Lymphocytic Leukemia |
Luminary Therapeutics, Inc., The Case Comprehensive Cancer Center, Case Western Reserve University |
Phase 1 |
| dual anti-CD19/anti-BAFF-R CAR T-cell therapy (Beckman Research Institute) |
BAFF-R x CD19 |
CAR-T |
Acute Lymphoblastic Leukemia |
Beckman Research Institute |
Preclinical |
| LMY-922 |
BAFF-R x BCMA x TACI |
Universal CAR-T |
Autoimmune Diseases |
Luminary Therapeutics, Inc. |
Preclinical |
| IL-18-secreting BCMA/BAFF-R targeting CAR T-cells (Cornell University) |
BAFF-R x BCMA x IL18R1 |
CAR-T |
Multiple Myeloma |
Cornell University |
Preclinical |
| BAFFRxCD3 BITE (Pepromene) |
BAFF-R x CD3 |
Bispecific T Cell Engager |
Tumors |
PeproMene Bio, Inc. |
Preclinical |
| LMY-921 |
BAFF-R x BCMA x TACI |
Universal CAR-T |
B-Cell Malignancies |
Luminary Therapeutics, Inc. |
Preclinical |
| LY-4152199 |
BAFF-R x CD3 |
Bispecific T Cell Engager |
B-Cell Malignancies |
Eli Lilly & Co. |
Preclinical |
| CD19/BAFF-R CAR T (City of Hope National Medical Center) |
BAFF-R x CD19 |
CAR-T |
Acute Lymphoblastic Leukemia |
City of Hope National Medical Center |
Preclinical |
| TH003-02 |
BAFF-R |
Cell Therapy |
B-Cell Lymphoma |
Bioneuritech (Shandong) Biopharmaceutical Group Co., Ltd. |
Preclinical |
| BAFF CAR-T cells (Case Western Reserve University) |
BAFF-R x BCMA x TACI |
CAR-T |
Hairy Cell Leukemia |
Case Western Reserve University School of Medicine |
Preclinical |
| BAFFR-Targeted CAR-NK (Pepromene) |
BAFF-R |
CAR-NK |
Tumors |
PeproMene Bio, Inc. |
Preclinical |
Overall, TNFRSF13C-targeted drugs show great potential in treating autoimmune diseases and B-cell malignancies by inhibiting B cell activation and eliminating abnormal B cells.
5. TNFRSF13C (BAFF-R) Related Products
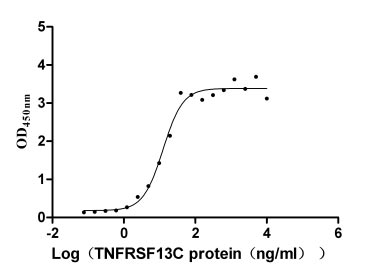
TNFRSF13C regulates B cell survival and immune homeostasis, playing a key role in immunodeficiency, autoimmune diseases, and tumors. CUSABIO introduces high-activity TNFRSF13C recombinant proteins, antibodies, and ELISA kits to support research into TNFRSF13C mechanisms and clinical applications.
References
[1] Smulski, C. R., & Eibel, H. (2018). BAFF and BAFF-Receptor in B Cell Selection and Survival. Frontiers in Immunology, 9, 2285.
[2] Sevdali, E., et al. (2021). BAFF receptor polymorphisms and deficiency in humans. Current Opinion in Immunology, 71, 103-110.
[3] Upadia, J., et al. (2018). A previously unrecognized 22q13.2 microdeletion syndrome that encompasses TCF20 and TNFRSF13C. American Journal of Medical Genetics Part A, 176 (12), 2791-2797.
[4] Losi, C. G., et al. (2005). Mutational analysis of human BAFF receptor TNFRSF13C (BAFF-R) in patients with common variable immunodeficiency. Journal of Clinical Immunology, 25 (5), 496-502.
[5] Warnatz, K., et al. (2009). B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proceedings of the National Academy of Sciences, 106 (33), 13945-13950.
[6] Lipsky, P. E., et al. (2022). Biological impact of iberdomide in patients with active systemic lupus erythematosus. Annals of the Rheumatic Diseases, 81 (9), 1136-1142.
[7] Parameswaran, R., et al. (2014). Effector-mediated eradication of precursor B acute lymphoblastic leukemia with a novel fc-engineered monoclonal antibody targeting the BAFF-R. Molecular Cancer Therapeutics, 13 (7), 1567-1577.
CUSABIO team. TNFRSF13C (BAFF-R): A Key Target for Immune Regulation and Disease Treatment. https://www.cusabio.com/c-21220.html





Comments
Leave a Comment