1. What is MICA
MICA (Major Histocompatibility Complex Class I-Related Chain A) is an important stress-inducing cell surface glycoprotein belonging to the MHC class I related protein family. The gene is located in the MHC region of human chromosome 6, immediately adjacent to the HLA-B locus, and the encoded protein consists of α1, α2, and α3 domains, as well as transmembrane and intracellular regions. MICA proteins are predominantly expressed on the surface of epithelial cells, endothelial cells, and certain tumor cells, but not in normal peripheral blood lymphocytes [1,2].
As the primary ligand of the NKG2D (Natural Killer Group 2D) receptor, MICA plays a key role in immune surveillance. NKG2D is widely expressed on the surface of NK cells, CD8+ T cells, and γδ T cells, and when MICA binds to NKG2D, it activates cytotoxicity in these cells and induces killing of abnormal cells [3,4]. Notably, the MICA gene has a high degree of polymorphism, with more than 400 alleles reported, which may affect its binding affinity and strength of immune response to NKG2D [5,6].
2. Mechanism of action and signaling pathways of MICA
2.1 Expression regulation and shedding mechanism of MICA
The expression of MICA is regulated by cellular stress signals, such as DNA damage, oxidative stress, or malignant transformation. In tumor cells, genomic instability often leads to transcriptional activation of the MICA gene, resulting in high surface expression [7,8]. However, tumor cells can cleave the MICA molecule by proteolysis, causing it to detach from the cell surface to form soluble MICA (sMICA). Studies have shown that metalloproteinases such as ADAM10 and ADAM17 are involved in this process, with cleavage sites located in the α3 domain of MICA [2,8]. sMICA not only reduces the density of NKG2D ligands on the surface of tumor cells, but also binds to circulating NKG2D, leading to receptor internalization and degradation, thereby weakening the antitumor activity of NK cells and T cells [4,8].
2.2 NKG2D-MICA signaling pathway
The signaling pathway initiated by the binding of NKG2D to MICA is mainly dependent on the adaptor protein DAP10 (DNAX-Activating Protein of 10 kDa). DAP10 contains an ITAM (Immunoreceptor Tyrosine-Based Activation Motif)-like motif that, after binding to NKG2D, activates downstream MAPK (Mitogen-Activated Protein Kinase) and MAPK (Mitogen-Activated Protein Kinase) by recruiting PI3K (Phosphatidylinositol 3-Kinase) and Akt kinase The NF-κB (Nuclear Factor kappa-B) signaling pathway ultimately promotes the release of cytotoxic molecules (e.g., perforin, granzyme) and the secretion of cytokines (e.g., IFN-γ) [3,4].
In NK cells, the balance of this signaling pathway with inhibitory receptors such as KIRs determines the activation status of NK cells. When MICA is highly expressed and inhibitory signals are weak, NK cells are fully activated to exert antitumor effects [8]. In the tumor microenvironment, the accumulation of sMICA can competitively bind to NKG2D, disrupting this signaling pathway and leading to immune escape [2,8].
3. MICA-related diseases
3.1 Malignant tumors
● Breast cancer: In triple-negative breast cancer (TNBC), the expression levels of MICA and MICB were positively correlated with NK cell infiltration. Elevated serum levels of sMICA in TNBC patients have been found to be associated with poor prognosis, and miR-486-5p can enhance NK cell killing of tumor cells by targeting MICA expression [7,9].
● Kidney cancer and melanoma: high expression of MICA on the surface of kidney cancer cells can be a target for NK cell attack, but tumor cells evade immune surveillance by shedding MCA. In melanoma, MICA expression is strongly associated with tumor progression and metastasis [8,10].
● Other tumors: MICA is aberrantly expressed in a variety of solid tumors, such as colorectal, ovarian, and pancreatic cancers, and its shedding levels can be used as biomarkers to assess tumor progression and prognosis [1,2].
3.2 Organ transplant rejection
Kidney transplant studies have shown that MICA mismatches between donors and recipients are significantly associated with reduced survival of transplanted kidneys. Patients with preoperative anti-MICA donor-specific antibodies (DSAs) have a significantly increased incidence of postoperative antibody-mediated rejection (ABMR), while new postoperative anti-MICA DSAs are associated with an increased risk of transplanted kidney failure [2,6]. In addition, there is a synergistic effect between MICA antibodies and HLA antibodies in transplant rejection, and the risk of ABMR is significantly increased when the two are co-existing [6].
4. Advances in drug research based on MICA targets
In February 2025, the mRNA vaccine mCM10-L, which targets the MICA/B α3 epitope, developed by a team from Zhejiang University, was published in Cell Reports Medicine, which significantly inhibits a variety of tumor metastasis by blocking MICA/B α1/2 shedding to activate immune cells [11]. At the same time, a number of MICA targeted drugs are in the preclinical or clinical research stage, with solid tumors as the main indication. Some of the pipelines under development are listed in the following table:
| Drugs |
Mechanism of action |
Type of medication |
Indications under investigation (disease name) |
Institutions under research |
Highest R&D stage |
| DM-919 |
MICA inhibitor | MICB inhibitors |
Monoclonal antibodies |
Advanced malignant solid tumors |
Danma (Suzhou) Biomedical Technology Co., Ltd |
Phase 1 clinical trial |
| CLN-619 |
MICA inhibitor | MICB inhibitors |
Monoclonal antibodies |
Recurrent multiple myeloma | Advanced malignant solid tumors | Non-small cell lung cancer | pancreatic cancer |
Cullinan Oncology LLC |
Phase 1 clinical trial |
| AHA-1031 |
MICA inhibitor | MICB inhibitors |
Bispecific antibodies |
STK11-mutated non-small cell lung cancer |
The University of Texas Southwestern Medical Center | Aakha Biologics | Alloy Therapeutics, Inc. |
Preclinical |
| FT-836 |
MICA inhibitor | MICB inhibitors |
CAR-T |
Solid tumors | tumor |
Fate Therapeutics, Inc. | Dana-Farber Cancer Institute, Inc. |
Preclinical |
| MICA/BxCD3(Xencor Inc.) |
CD3 stimulator | MICA inhibitor | MICB inhibitors |
Bispecific T cell binder |
tumor |
Xencor, Inc. |
Preclinical |
| SYB-010 |
MICA inhibitor | MICB inhibitors |
Monoclonal antibodies |
tumor |
CanCure LLC |
Preclinical |
| Tri-modal CAR+TCR+hnCD16+iPSC-derived T cells(Fate) |
BCMA inhibitor | CD16a modulator | MICA inhibitor | MICB inhibitor | NY-ESO-1 inhibitors |
Induced pluripotent stem cells | CAR-T |
Solid tumors |
Fate Therapeutics, Inc. |
Preclinical |
| JZC01 |
MICA inhibitor | VEGFR2 antagonists |
Bispecific antibodies |
tumor |
China Pharmaceutical University |
Preclinical |
| BSI-120 |
MICA inhibitor | MICB inhibitors |
Monoclonal antibodies |
tumor |
Biosion Biotechnology (Nanjing) Co., Ltd |
Preclinical |
| IPH4301 |
DNA inhibitors | MICA inhibitors |
ADC |
tumor |
Innate Pharma SA |
Preclinical |
| IPH-43 |
DNA inhibitors | MICA inhibitors |
ADC |
Solid tumors |
AstraZeneca PLC |
Preclinical |
| B10G5 |
MICA inhibitor | MICB inhibitor | Natural killer cell modulator |
Monoclonal antibodies |
Multiple myeloma | Metastatic prostate cancer |
Severance Hospital | CanCure LLC |
Preclinical |
| ADI-925 |
MICA inhibitor | MICB inhibitor | ULBP1 inhibitors |
General purpose CAR-T |
Solid tumors |
Adicet Therapeutics, Inc. |
Preclinical |
| PDL1sFv/MICAe |
MICA inhibitor | PDL1 inhibitors |
Antibodies | Fusion proteins |
tumor |
Clemson University |
Preclinical |
| GenSci-P107 |
MICA inhibitor | MICB inhibitors |
Bispecific antibodies |
Hepatocellular carcinoma | Non-small cell lung cancer | Colorectal cancer | gastric cancer |
Changchun Jinsai Pharmaceutical Co., Ltd |
Preclinical |
5. CUSABIO related product recommendation
As a key ligand for NKG2D, MICA plays an important role in tumor immune surveillance and transplant rejection. Its unique expression regulation and shedding mechanism provide a target for the development of novel immunotherapy drugs. Huamei Biotech provides MICA proteins, antibodies, and ELISA kits to help you research the mechanism of immunosuppressive action of MICA or drug development.
References
[1] Zou Y, et al. Antibodies against MICA antigens and kidney-transplant rejection. The New England Journal of Medicine, 2007.
[2] Carapito R, et al. The MHC class I MICA gene is a histocompatibility antigen in kidney transplantation. Nature Medicine, 2022.
[3]Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science, 1999.
[4] Raulet DH, et al. Regulation of ligands for the NKG2D activating receptor. Annual Review of Immunology, 2013.
[5] Klussmeier A, et al. High-throughput MICA/B genotyping of over two million samples: workflow and allele frequencies. Frontiers in Immunology, 2020.
[6] Zou Y, et al. Antibodies against MICA antigens and kidney-transplant rejection: reply. The New England Journal of Medicine, 2008.
[7] Abdel-Latif M, et al. A new quercetin glycoside enhances TNBC immunological profile through TP53/miR-155/MICA/ULBP2. Annals of Oncology, 2019.
[8] Ferrari de Andrade L, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science, 2018.
[9] Elkhouly A, et al. miR-486-5p counteracts the shedding of MICA/B and CD155 immune-ligands in TNBC patients. Annals of Oncology, 2019.
[10] Badrinath S, et al. Promoting T and NK cell attack: preserving tumor MICA/B by vaccines. Cell Research, 2022.
[11] Rui Wang, et al. An epitope-directed mRNA vaccine inhibits tumor metastasis through the blockade of MICA/B α1/2 shedding. Cell Rep Med, 2025.
CUSABIO team. MICA: key regulatory molecule for tumor immune surveillance and its therapeutic target potential. https://www.cusabio.com/c-21243.html

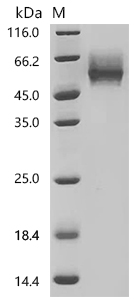
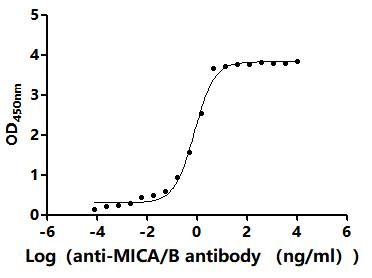
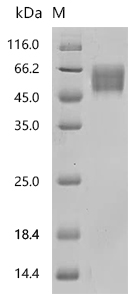
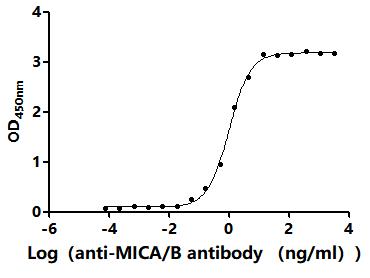


Comments
Leave a Comment