1. What is PTH
Parathyroid hormone (PTH) is the principal hormone secreted by the parathyroid glands and is essential for maintaining calcium-phosphate homeostasis. It critically regulates bone metabolism, renal calcium re-absorption, and intestinal calcium absorption [1]. PTH secretion is negatively regulated by serum calcium via the calcium-sensing receptor (CaSR) and is further modulated by vitamin D and phosphate [2]. Its mechanism of action is intricate, involving multiple signaling cascades and associating with a spectrum of pathologies. These actions are chiefly mediated through the PTH1R receptor, governing bone metabolism, mineral balance, and the onset and progression of related disorders.
2. Research Mechanisms of PTH
2.1 Regulation of Bone Metabolism
PTH modulates bone turnover by directly targeting osteoblasts and osteoclasts. Intermittent PTH administration activates anabolic pathways in osteoblasts, promoting bone formation by increasing osteoblast number, delaying apoptosis, and enhancing matrix deposition [3]. Under PTH stimulation, osteoblasts meet the energetic demands of collagen synthesis via aerobic glycolysis and oxidative phosphorylation [3]. In addition, PTH triggers lipolysis in bone-marrow adipocytes (BMAdipo), releasing fatty acids to fuel osteogenesis [3].
2.2 Calcium-Phosphate Homeostasis
In the kidney, PTH enhances calcium re-absorption and suppresses phosphate re-absorption while stimulating synthesis of 1,25-dihydroxyvitamin D, thereby indirectly augmenting intestinal calcium uptake [3]. Within the parathyroid gland, CaSR senses serum calcium to govern PTH release. The γ-aminobutyric acid B1 receptor (GABAB1R) heterodimerizes with CaSR, negatively modulating CaSR signaling and fostering excessive PTH secretion—a mechanism pivotal in hyperparathyroidism [2].
2.3 Interactions with Other Molecules
The uremic toxin indoxyl sulfate (IS) interacts with PTH to alter osteocyte function. In mature osteocytes, IS up-regulates bone-resorption-associated genes (e.g., RANKL/OPG) and oxidative-stress genes (NOX1, NOX4), whereas PTH down-regulates bone-formation-inhibitory genes (SOST, Dkk1); the two molecules influence bone remodeling through distinct mechanisms [4]. During osteocyte mineralization, IS and PTH synergistically inhibit alkaline phosphatase activity, thereby impeding mineral deposition [4].
3. PTH Signaling Pathways
3.1 Canonical PTH1R-Mediated Pathways
PTH primarily signals through PTH1R, a G-protein-coupled receptor (GPCR):
- cAMP/PKA pathway: The predominant route; PTH binding activates adenylyl cyclase, elevating cAMP and activating PKA to regulate expression of bone-formation-related genes such as IGF-1 and FGF2 [3,5].
- PLC/PKC pathway: High PTH concentrations activate phospholipase C (PLC), hydrolyzing PIP2 to IP3 and DAG. IP3 mobilizes intracellular calcium, while DAG activates PKC, participating in osteocytic differentiation [3].
3.2 Heteroreceptor Interactions
CaSR and GABAB1R form heterodimers in parathyroid cells. GABA, released in an autocrine fashion, binds this complex, dampening CaSR sensitivity to calcium and weakening the negative feedback on PTH secretion, thus promoting excessive PTH release [2].
3.3 Interference and Blockade of Signaling
PTH primarily signals through PTH1R, a G-protein-coupled receptor (GPCR):
- Immunoassay interference: Murine antibodies (e.g., oregovomab) can cross-react with anti-mouse antibodies in assay reagents, causing spuriously elevated PTH values [6].
- Autoantibody blockade: Autoantibodies targeting the extracellular domain of PTH1R prevent PTH binding and downstream cAMP signaling, leading to PTH resistance and hypocalcemia [5].
4. PTH-Related Disorders
4.1 Primary Hyperparathyroidism
Autonomous parathyroid lesions (adenoma, hyperplasia, or carcinoma) drive excessive PTH secretion. Excess PTH activates osteoclasts, accelerates bone resorption, and raises serum calcium while suppressing renal phosphate re-absorption, resulting in hypophosphatemia. Chronic hypercalcemia may manifest as bone pain, nephrolithiasis, and gastrointestinal disturbances [7].
4.2 Secondary Hyperparathyroidism (SHPT)
SHPT is common in chronic kidney disease (CKD). Declining renal function impairs phosphate excretion; elevated phosphate complexes with calcium, lowering serum calcium. Diminished renal synthesis of 1,25-dihydroxyvitamin D further reduces intestinal calcium absorption. Persistent hypocalcemia stimulates parathyroid hyperplasia and compensatory PTH hypersecretion, creating a vicious cycle [8].
4.3 Postoperative Hypoparathyroidism
Surgical removal or devascularization of parathyroid glands during thyroid or parathyroid surgery impairs PTH secretion. PTH deficiency attenuates osteoclast activity and renal calcium re-absorption, leading to hypocalcemia with tetany, paresthesia, and, over time, altered bone mineral density [9].
4.4 PTH-Resistance Syndromes
Caused by autoantibodies against PTH1R or genetic defects. Autoantibodies block PTH binding and downstream cAMP signaling, yielding hypocalcemia, hyperphosphatemia, and elevated PTH. Patients present with muscle cramps and sensory disturbances [5].
5. Recent Advances in PTH-Targeted Therapeutics
Recent years have witnessed significant progress in PTH-targeted drug development, spanning small molecules, antibodies, and novel mechanisms. Cutting-edge research focuses on precise modulation of PTH1R and innovative targeting of PTHrP pathways. Molecular-dynamics simulations have identified mechanostructural allosteric sites on PTH1R, enabling the first negative allosteric and signal-biased small molecules to enter pre-clinical optimization, potentially overcoming GPCR allosteric-drug bottlenecks. Meanwhile, the PTHrP-targeting antibody BGM-2121, which recognizes the N-terminal (1-34) epitope, simultaneously suppresses tumor growth, reverses hypercalcemia, and ameliorates cachexia in pancreatic-cancer models. Having completed safety and pharmacokinetic validation, it is poised to enter clinical trials, offering a novel therapeutic avenue for PTHrP-overexpressing tumors.
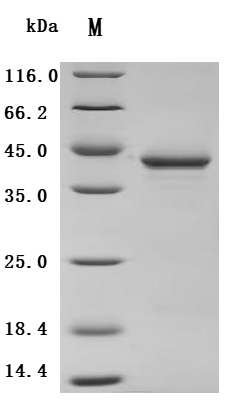
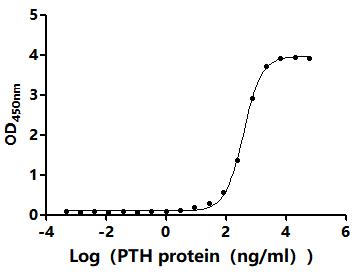
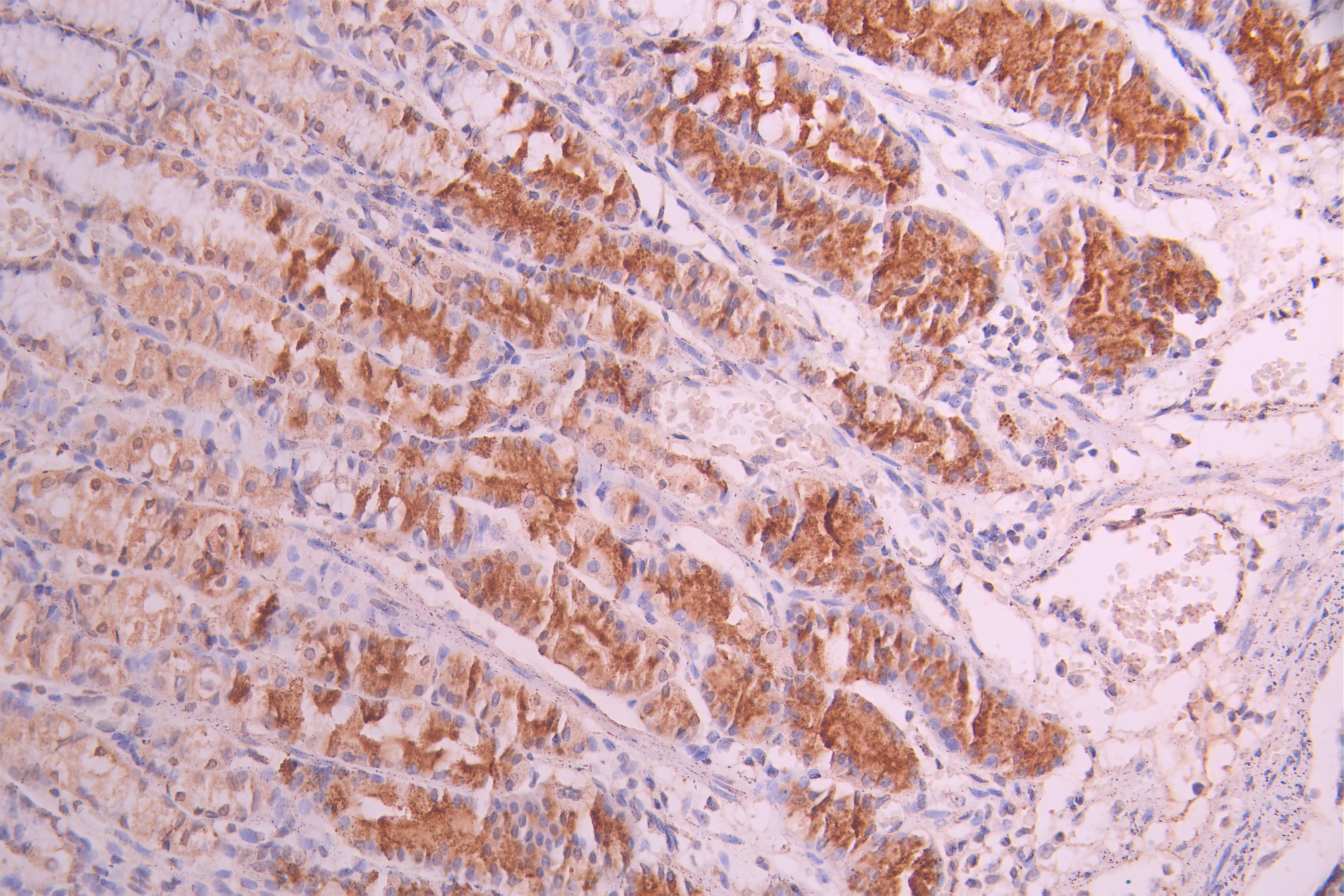
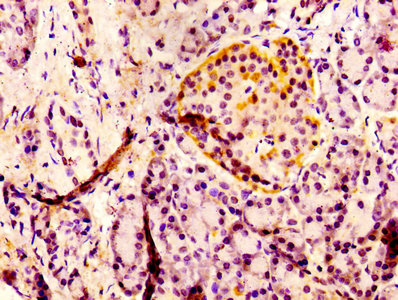
6. Recommended PTH Research Products
CUSABIO supplies recombinant PTH proteins, antibodies, and ELISA kits to support your PTH mechanism studies and drug development.
References
[1] Cavalcante L B C P, Brandão C M Á, Chiamolera M I, et al. Big data-based parathyroid hormone (PTH) values emphasize need for age correction[J]. Journal of Endocrinological Investigation, 2023, 46: 2525-2533.
[2] Starling S. Insights into parathyroid hormone secretion[J]. Nature Reviews Endocrinology, 2020.
[3] Rendina-Ruedy E, Rosen C J. Parathyroid hormone (PTH) regulation of metabolic homeostasis: An old dog teaches us new tricks[J]. Molecular Metabolism, 2022, 60: 101480.
[4] Chen N X, O’Neill K, Moe S M. Uremic Toxin Indoxyl Sulfate (IS) and Parathyroid Hormone (PTH) Interact and Affect Osteocyte Signaling and Function[J]. J Am Soc Nephrol, 2023, 34.
[5] Mandl A, Burbelo P D, Di Pasquale G, et al. Parathyroid Hormone Resistance and Autoantibodies to the PTH1 Receptor[J]. New England Journal of Medicine, 2021, 385(21): 1974-1980.
[6] Mendpara V A, McShane A J, Khan L Z. 7337 High Parathyroid Hormone (PTH) Due To Immunoassay Interference[J]. Journal of the Endocrine Society, 2024, 8(Supplement_1): A226-A227.
[7] Cusano N E, Bilezikian J P. Parathyroid Hormone in the Evaluation of Hypercalcemia[J]. JAMA, 2014, 312(24): 2680-2681.
[8] Block G A, Bushinsky D A, Cheng S, et al. Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis With Secondary Hyperparathyroidism: A Randomized Clinical Trial[J]. JAMA, 2017, 317(2): 156-164.
[9] Hashem M, Lim C B, Balasubramanian S P. Postoperative parathyroid hormone (PTH) is equivalent to perioperative PTH drop in predicting postsurgical hypoparathyroidism[J]. Annals of the Royal College of Surgeons of England, 2024, 106: 547-552.
CUSABIO team. Parathyroid Hormone (PTH): A Key Target for Regulating Calcium-Phosphate Metabolism and Skeletal Health. https://www.cusabio.com/c-21250.html



Comments
Leave a Comment