[1] Michael Shapiro, Herut Dor, Anna Gurevich-Shapiro, Tal Etan, Ido Wolf.(2024). Institutional-Level Monitoring of Immune Checkpoint Inhibitor IrAEs Using a Novel Natural Language Processing Algorithmic Pipeline.
[2] Kamran Kaveh, Feng Fu.(2021). Immune checkpoint therapy modeling of PD-1/PD-L1 blockades reveals subtle difference in their response dynamics and potential synergy in combination.
[3] Qinchuan Wang, Yue He, Wanlu Li, Xiaohang Xu, Qingfeng Hu, Zilong Bian, A. Xu, H. Tu, Ming Wu, Xifeng Wu.(2022). Soluble Immune Checkpoint-Related Proteins in Blood Are Associated With Invasion and Progression in Non-Small Cell Lung Cancer.
[4] B. Rapoport, R. Anderson, N. Malinga, H. Steel, P. Meyer, T. Smit, M. Kgokolo.(2023). Transforming growth factor-b1 and soluble co-inhibitory immune checkpoints as putative drivers of immune suppression in advanced basal cell carcinoma.
[5] A. Desai, V. Subbiah, A. Dimou, J. Deshane, Kayla F. Goliwas, S. Ponnazhagan, D. Das, M. Khalil, Y. Lo, Edwin Lin.(2023). Exploring the potential of combination immune checkpoint strategies in non-small cell lung cancer (NSCLC).
[6] Chun Zeng, Tinghe Wu, Y. Zhen, X. Xia, Yong Zhao.(2005). BTLA, a new inhibitory B7 family receptor with a TNFR family ligand.
[7] W. Hobo, W. J. Norde, N. Schaap, H. Fredrix, F. Maas, Karen Schellens, J. Falkenburg, A. Korman, D. Olive, R. van der Voort, H. Dolstra.(2012). B and T Lymphocyte Attenuator Mediates Inhibition of Tumor-Reactive CD8+ T Cells in Patients After Allogeneic Stem Cell Transplantation.
[8] Adeolu O. Adegoke, G. Thangavelu, Ting-Fang Chou, Marcos I. Petersen, K. Kakugawa, J. May, K. Joannou, Qingyang Wang, K. K. Ellestad, Louis Boon, Peter A. Bretscher, H. Cheroutre, Mitchell Kronenberg, T. Baldwin, Colin C. Anderson.(2024). Internal regulation between constitutively expressed T cell co-inhibitory receptors BTLA and CD5 and tolerance in recent thymic emigrants.
[9] Andrew C Vendel, L. Jaroszewski, Matthew D Linnik, Adam Godzik.(2024). B‐ and T‐Lymphocyte Attenuator in Systemic Lupus Erythematosus Disease Pathogenesis.
[10] Christian Sordo-Bahamonde, Seila Lorenzo-Herrero, Alejandra G Martinez-Perez, A. P. Gonzalez-Rodriguez, Á. Payer, E. González-García, Candelaria Aguilar-García, Sara González-Rodríguez, A. López-Soto, A. García-Torre, S. González.(2023). BTLA dysregulation correlates with poor outcome and diminished T cell-mediated antitumor responses in chronic lymphocytic leukemia.
[11] Mie Oki, N. Watanabe, T. Owada, Yoshihiro Oya, K. Ikeda, Y. Saito, R. Matsumura, Y. Seto, I. Iwamoto, H. Nakajima.(2011). A Functional Polymorphism in B and T Lymphocyte Attenuator Is Associated with Susceptibility to Rheumatoid Arthritis.
[12] P. Guruprasad, A. Carturan, Yunlin Zhang, K. G. Kumashie, Ivan J Cohen, G. Ghilardi, Ki-Hyun Kim, Jong-Seo Lee, Yoon Lee, Jong-Hoon Kim, J. Chung, Maksim Shestov, R. Pajarillo, Jaryse Harris, Yong Gu Lee, Michael Wang, H. Ballard, Aasha Gupta, O. Ugwuanyi, S. Hong, Linhui Chen, L. Paruzzo, Shane C Kammerman, R. Patel, O. Shestova, L. Vella, S. Schuster, J. Svoboda, P. Porazzi, M. Ruella.(2023). Modulation of the Btla-HVEM Axis to Enhance CAR T Cell Immunotherapy Against Cancer.
[13] Xiaozheng Xu, T. Masubuchi, Qixu Cai, Yunlong Zhao, E. Hui.(2021). Molecular features underlying differential SHP1/SHP2 binding of immune checkpoint receptors.
[14] J. Chemnitz, R. Parry, K. Nichols, C. June, J. Riley.(2004). SHP-1 and SHP-2 Associate with Immunoreceptor Tyrosine-Based Switch Motif of Programmed Death 1 upon Primary Human T Cell Stimulation, but Only Receptor Ligation Prevents T Cell Activation1.
[15] Xiangmin Li, Zhaoguo Xu, Guoyuan Cui, Li Yu, Xiaoye Zhang.(2020). BTLA Expression in Stage I–III Non–Small-Cell Lung Cancer and Its Correlation with PD-1/PD-L1 and Clinical Outcomes.
[16] Minglei Yang, Chenxi Zheng, Yu Miao, Cuicui Yin, Longfei Tang, Chongli Zhang, Pu Yu, Qingfang Han, Yihui Ma, Shenglei Li, Guozhong Jiang, Wencai Li, Peiyi Xia.(2025). BTLA promoter hypomethylation correlates with enhanced immune cell infiltration, favorable prognosis, and immunotherapy response in melanoma.
[17] Suzanne Mélique, Aurélie Vadel, Nelly Rouquié, Cui Yang, Cyrielle Bories, Coline Cotineau, Abdel Saoudi, N. Fazilleau, Renaud Lesourne.(2024). THEMIS promotes T cell development and maintenance by rising the signaling threshold of the inhibitory receptor BTLA.
[18] Shane Atwell, T. Cheung, Elaine M Conner, Carolyn Ho, Jiawen Huang, Erin L Harryman, Ricky Lieu, Stacie Lim, Wai W Lin, Diana I Ruiz, Andrew C Vendel, Carl F Ware.(2025). Quantitative detection of the HVEM-BTLA checkpoint receptor cis-complex in human lymphocytes.
[19] N. Aubert, S. Brunel, D. Olive, G. Marodon.(2021). Blockade of HVEM for Prostate Cancer Immunotherapy in Humanized Mice.
[20] C. Demerlé, L. Gorvel, M. Mello, S. Pastor, C. Degos, A. Zarubica, F. Angelis, F. Fiore, J. Nunès, B. Malissen, L. Greillier, G. Guittard, H. Luche, F. Barlesi, D. Olive.(2023). Anti-HVEM mAb therapy improves antitumoral immunity both in vitro and in vivo, in a novel transgenic mouse model expressing human HVEM and BTLA molecules challenged with HVEM expressing tumors.
[21] Karolina Wojciechowicz, Katarzyna Kuncewicz, Jacek Rutkowski, Jacek Jassem, Anna Wardowska, M. Spodzieja.(2024). The effect of gD-derived peptides on T cell immune response mediated by BTLA-HVEM protein complex in melanoma patients.
[22] C. Battin, J. Leitner, Petra Waidhofer-Söllner, K. Grabmeier-Pfistershammer, D. Olive, P. Steinberger.(2022). BTLA inhibition has a dominant role in the cis-complex of BTLA and HVEM.
[23] R. Flynn, Tarun E. Hutchinson, K. Murphy, C. Ware, M. Croft, Shahram Salek-Ardakani.(2013). CD8 T Cell Memory to a Viral Pathogen Requires Trans Cosignaling between HVEM and BTLA.
[24] Bin Yang, Zhuochun Huang, Wei-hua Feng, Wei Wei, Junlong Zhang, Y. Liao, Linhui Li, Xinle Liu, Zhiqiang Wu, B. Cai, Yang-juan Bai, Lanlan Wang.(2016). The Expression of LIGHT Was Increased and the Expression of HVEM and BTLA Were Decreased in the T Cells of Patients with Rheumatoid Arthritis.
[25] Weiwei He, Jing Zhao, Xue-rong Liu, Sheli Li, K. Mu, Jing Zhang, Jin-an Zhang.(2020). Associations between CD160 polymorphisms and autoimmune thyroid disease: a case-control study.
[26] Yanbo Kou, Xingping Zheng, Liyuan Meng, Mengnan Liu, Shihong Xu, Qiyue Jing, Shenghan Zhang, Hanying Wang, Jinzhi Han, Zhuanzhuan Liu, Yanxia Wei, Yugang Wang.(2022). The HVEM-BTLA Immune Checkpoint Restrains Murine Chronic Cholestatic Liver Injury by Regulating the Gut Microbiota.
[27] B. Érsek, P. Silló, Uğur Çakır, V. Molnar, A. Bencsik, Balázs Mayer, É. Mezey, S. Kárpáti, Z. Pós, K. Nemeth.(2020). Melanoma-associated fibroblasts impair CD8+ T cell function and modify expression of immune checkpoint regulators via increased arginase activity.
[28] L. Gorvel, C. Demerlé, M. Mello, S. Pastor, C. Degos, A. Zarubica, F. Angelis, Frederic Fiore, Jacques A. Nunès, Bernard Malissen, Laurent Greillier, G. Guittard, Hervé Luche, F. Barlesi, Daniel Olive.(2023). Abstract 5184: Anti-HVEM mAb therapy improves antitumoral immunity both in vitro and in vivo, in a novel transgenic mouse model expressing human HVEM and BTLA molecules challenged with HVEM expressing tumors.
[29] Chong-bin Hu, Chen Huang, Jie Wang, Yun Hong, Dong-dong Fan, Ye Chen, Ai-fu Lin, L. Xiang, J. Shao.(2023). Novel PD-L1/BTLA Checkpoint Axis Exploited for Bacterial Immune Escape by Restraining CD8+ T Cell-Initiated Adaptive Immunity in Zebrafish.
[30] Ryohei Kondo, Kohei Kondo, Kei Nabeshima, Akihiko Nishikimi, Y. Ishida, Toshiaki Shigeoka, Johannes M. Dijkstra.(2025). PD-1 is conserved from sharks to humans: new insights into PD-1, PD-L1, PD-L2, and SHP-2 evolution.
[31]Zhigang Rong, Fei Zhang, Zhengdong Wang, Weifeng He, S. Dong, Jianzhong Xu, F. Dai.(2018). Improved Osteogenesis by HVEM-Expressing Allogenic Bone Marrow-Derived Mesenchymal Stem Cells in an Immune Activation Condition and Mouse Femoral Defect Model.
[32] M. Marasco, Anna Berteotti, J. Weyershaeuser, N. Thorausch, Justyna Sikorska, J. Krausze, H. Brandt, Joanna Kirkpatrick, P. Ríos, W. Schamel, M. Köhn, T. Carlomagno.(2020). Molecular mechanism of SHP2 activation by PD-1 stimulation.
[33] Ling Liu, Yan Cheng, Zhigang Zhang, Jing Li, Y. Geng, Qingsong Li, D. Luo, Li Liang, Wei Liu, Jianping Hu, W. Ouyang.(2023). Study on the allosteric activation mechanism of SHP2 via elastic network models and neural relational inference molecular dynamics simulation.
[34] C. Foster, Jasper Du, Oscar Pundel, M. Geer, Ryan C. Ripert, Jia Liu, Taylor A. Heim, K. Araki, Amanda W. Lund, Jun Wang, Ben Neel.(2025). T lymphocyte-specific deletion of SHP1 and SHP2 promotes activation-induced cell death of CD4+ T cells and impairs antitumor response.
[35] Christine Quach, Xin Li, Pedram Shafiei-Jahani, Meng Li, Stephen Shen, D. Helou, Benjamin P. Hurrell, P. Soroosh, Omid Akbari.(2025). BTLA agonist attenuates Th17-driven inflammation in a mouse model of steroid-resistant asthma.
[36] Jingjing Song, Lihui Wu.(2020). Friend or Foe: Prognostic and Immunotherapy Roles of BTLA in Colorectal Cancer.
[37] P. Diefenhardt, M. Braumann, Thomas Schömig, Bastian Trinsch, Claudio Sierra Gonzalez, J. Becker-Gotot, L. Völker, Lioba Ester, Amrei M. Mandel, D. Hawiger, Ali T. Abdallah, B. Schermer, H. Göbel, P. Brinkkötter, C. Kurts, T. Benzing, Sebastian Brähler.(2023). Stimulation of Immune Checkpoint Molecule B and T-Lymphocyte Attenuator Alleviates Experimental Crescentic Glomerulonephritis.
[38] F. Anzengruber, D. Ignatova, T. Schlaepfer, Yun-Tsan Chang, L. French, S. Pascolo, E. Contassot, M. Bobrowicz, W. Hoetzenecker, E. Guenova.(2019). Divergent LAG-3 versus BTLA, TIGIT, and FCRL3 expression in Sézary syndrome.
[39] A. Ruggieri, M. Yarchoan, S. Goyal, Yuan Liu, E. Sharon, Helen X. Chen, Brian M Olson, C. Paulos, B. El-Rayes, S. Maithel, N. Azad, G. Lesinski.(2022). Combined MEK/PD-L1 inhibition alters peripheral cytokines and lymphocyte populations correlating with improved clinical outcomes in advanced biliary tract cancer.
[40] W. Truong, J. C. Plester, W. Hancock, S. Merani, T. Murphy, K. Murphy, J. Kaye, C. Anderson, A. M. Shapiro.(2007). Combined Coinhibitory and Costimulatory Modulation with Anti‐BTLA and CTLA4Ig Facilitates Tolerance in Murine Islet Allografts.
[41] Radhika R. Gudi, Subha Karumuthil‐Melethil, Nicolas Pérez, Gongbo Li, C. Vasu.(2019). Engineered Dendritic Cell-Directed Concurrent Activation of Multiple T cell Inhibitory Pathways Induces Robust Immune Tolerance.
[42] Tony T. Jiang, O. Kruglov, G. Lin, Angela Minic, Kimberly R. Jordan, R. Uger, M. Wong, Y. Shou, O. Akilov.(2021). Clinical Response to Anti-CD47 Immunotherapy Is Associated with Rapid Reduction of Exhausted Bystander CD4+ BTLA+ T Cells in Tumor Microenvironment of Mycosis Fungoides.
[43] A. Kosmaczewska, L. Ciszak, Anna Andrzejczak, A. Tomkiewicz, Anna Partyka, Zofia Rojek-Gajda, Irena Frydecka, Dariusz Wołowiec, Tomasz Wróbel, A. Bojarska-Junak, Jacek Roliński, Lidia Karabon.(2025). miR-155-5p Silencing Does Not Alter BTLA Molecule Expression in CLL T Cells: Implications for Targeted Immunotherapy.
[44] D. Nishizaki, Sharon Choi, Chinmayi Pandya, Suzanna Lee, S. Pabla, P. DePietro, Taylor J. Jensen, R. Kurzrock, S. Kato.(2025). Pan-Cancer Landscape of B- and T-Lymphocyte Attenuator: Implications for Potential Immunotherapy Combinations.
[45] Binghao Li, Qinchuan Wang, Yihong Luo, Sicong Wang, Sai Pan, Wenting Zhao, Zhaoming Ye, Xifeng Wu.(2024). Peripheral Soluble Immune Checkpoint-Related Proteins Were Associated with Survival and Treatment Efficacy of Osteosarcoma Patients, a Cohort Study.
[46] Patrícia Neuperger, K. Szalontai, Nikolett Gémes, Jozsef A Balog, László Tiszlavicz, J. Furák, György Lázár, László G. Puskás, G. Szebeni.(2023). Single-cell mass cytometric analysis of peripheral immunity and multiplex plasma marker profiling of non-small cell lung cancer patients receiving PD-1 targeting immune checkpoint inhibitors in comparison with platinum-based chemotherapy.
[47] J. Ries, Leah Trumet, Alina Hahn, Lina Kunater, Rainer Lutz, C. Geppert, M. Kesting, Manuel Weber.(2024). The Immune Checkpoint BTLA in Oral Cancer: Expression Analysis and Its Correlation to Other Immune Modulators.
[48]Michelle E Wakeley, Brandon E Armstead, Chyna C. Gray, Elizabeth W. Tindal, Daithi S. Heffernan, C. Chung, A. Ayala.(2023). Lymphocyte HVEM/BTLA co-expression after critical illness demonstrates severity indiscriminate upregulation, impacting critical illness-induced immunosuppression.
[49]Julie Joseph, T. Premeaux, Daniel O. Pinto, Ahbishek Rao, Shrobona Guha, A. Panfil, A. Carey, L. Ndhlovu, E. Bergmann-Leitner, P. Jain.(2023). Extracellular immune checkpoint molecules released from HTLV-1-infected cells mount immune suppression in the context of neuroinflammation.
[50] Chien-Hsun Huang, Lei Cong, Jun Xie, Bo Qiao, S. Lo, T. Zheng.(2009). Rheumatoid arthritis-associated gene-gene interaction network for rheumatoid arthritis candidate genes.
[51] A. Small, S. Cole, J. J. Wang, S. Nagpal, Ling-Yang Hao, M. Wechalekar.(2022). Attenuation of the BTLA/HVEM Regulatory Network in the Circulation in Primary Sjögren’s Syndrome.
[52] Haruka Kinosada, Jun-ichirou Yasunaga, Kazuya Shimura, P. Miyazato, Chiho Onishi, T. Iyoda, K. Inaba, M. Matsuoka.(2017). HTLV-1 bZIP Factor Enhances T-Cell Proliferation by Impeding the Suppressive Signaling of Co-inhibitory Receptors.
[53] Kai Werner, S. Dolff, Yang Dai, Xin Ma, A. Brinkhoff, J. Korth, A. Gäckler, H. Rohn, Ming Sun, J. C. Cohen Tervaert, P. van Paassen, A. Kribben, O. Witzke, B. Wilde.(2019). The Co-inhibitor BTLA Is Functional in ANCA-Associated Vasculitis and Suppresses Th17 Cells.
[54] Bin Yang, Junlong Zhang, Lixin Li, Xiaojun Lyu, Wei Wei, Zhuochun Huang, B. Cai, Lanlan Wang.(2017). Genetic variations in LIGHT are associated with susceptibility to ankylosing spondylitis in a Chinese Han population.
[55] H. Douna, J. Amersfoort, F. Schaftenaar, M. Kröner, Máté G Kiss, B. Slütter, M. Depuydt, Mireia N A Bernabé Kleijn, A. Wezel, H. Smeets, H. Yagita, C. Binder, I. Bot, G. V. van Puijvelde, J. Kuiper, A. Foks.(2019). BTLA stimulation protects against atherosclerosis by regulating follicular B cells.
[56] P. Diefenhardt, Marie Braumann, Bastian Trinsch, Thomas Schömig, Claudio Sierra Gonzalez, Bernhard Schermer, Thomas Benzing, P. Brinkkoetter, Sebastian Braehler.(2022). Immune Checkpoint Molecule BTLA Attenuates Experimental Glomerulonephritis by Directly Inhibiting T Effector Cells and Inducing Treg Differentiation.

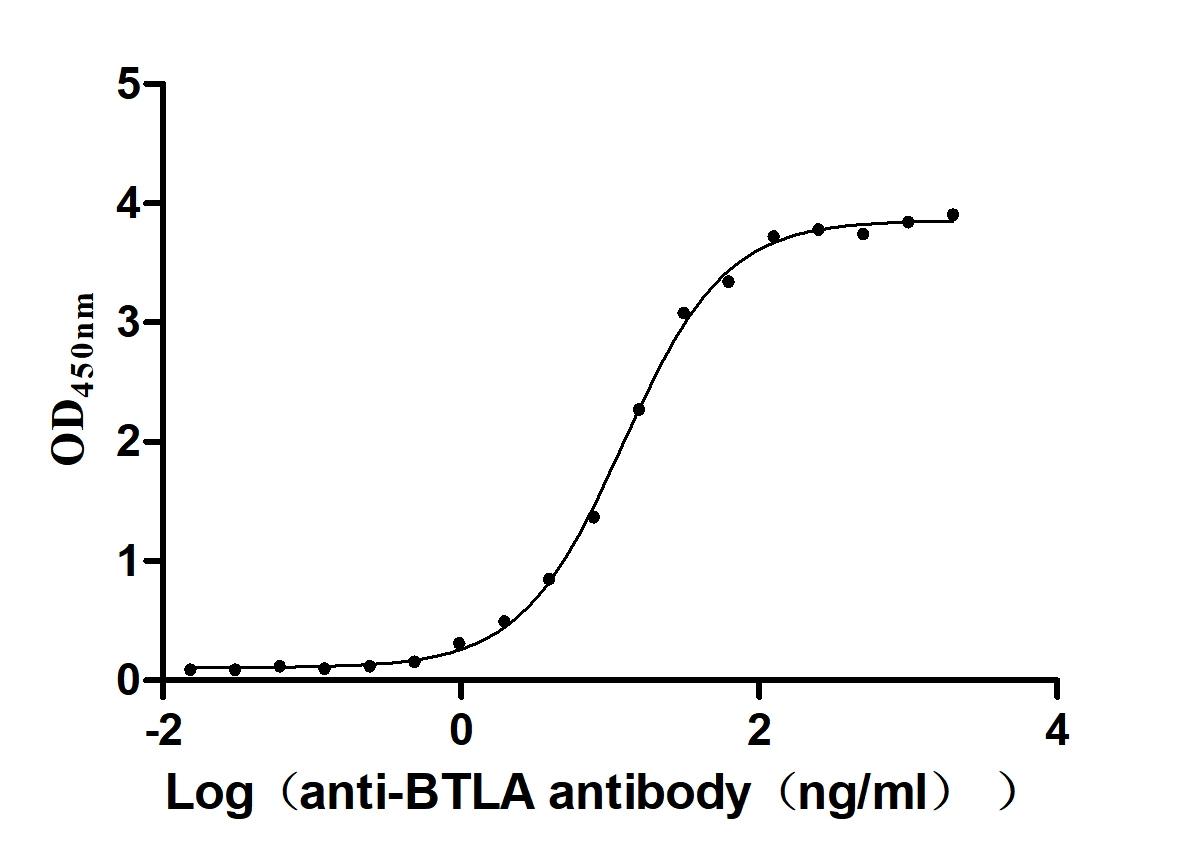
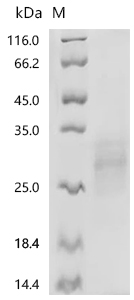
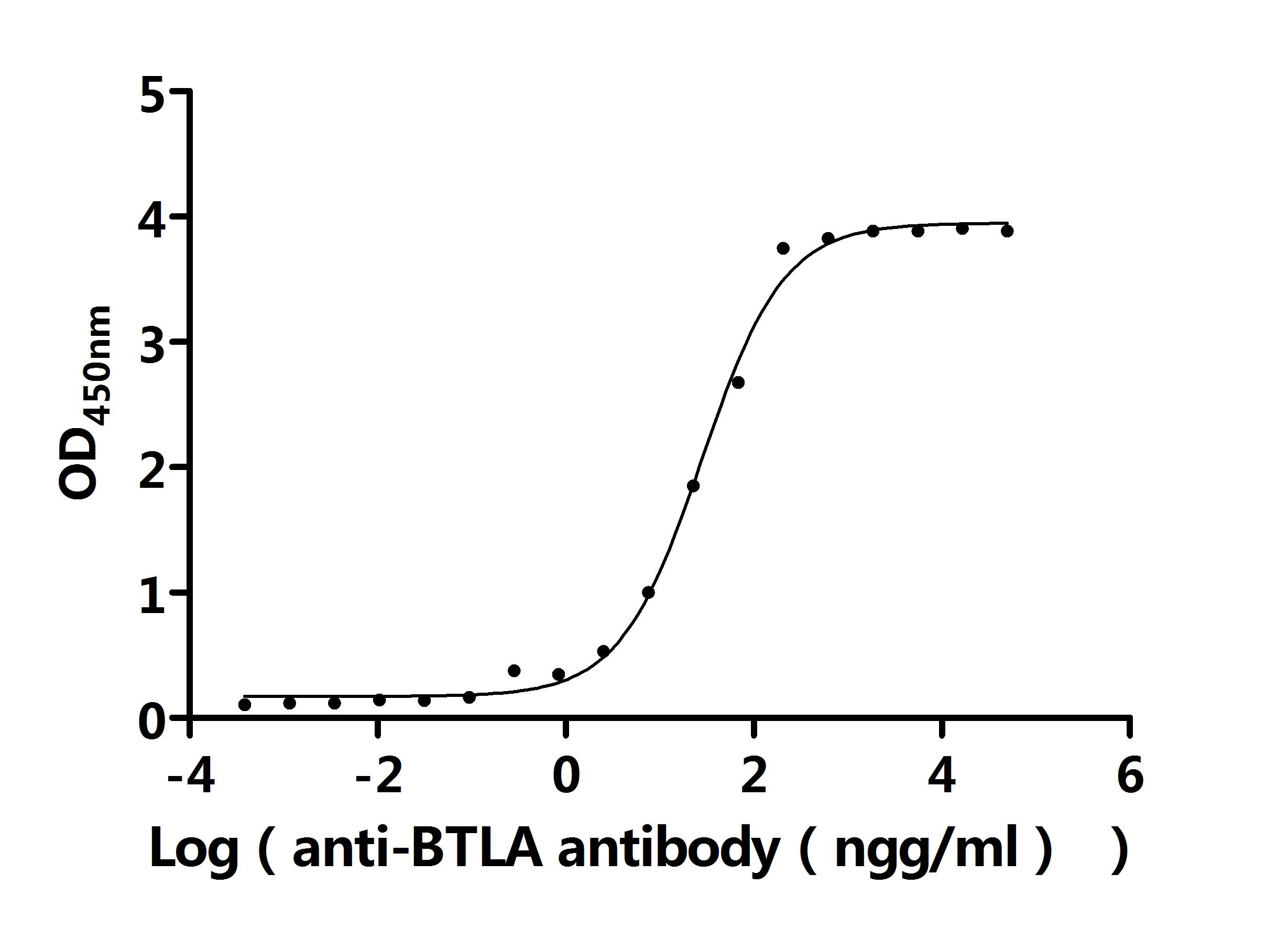
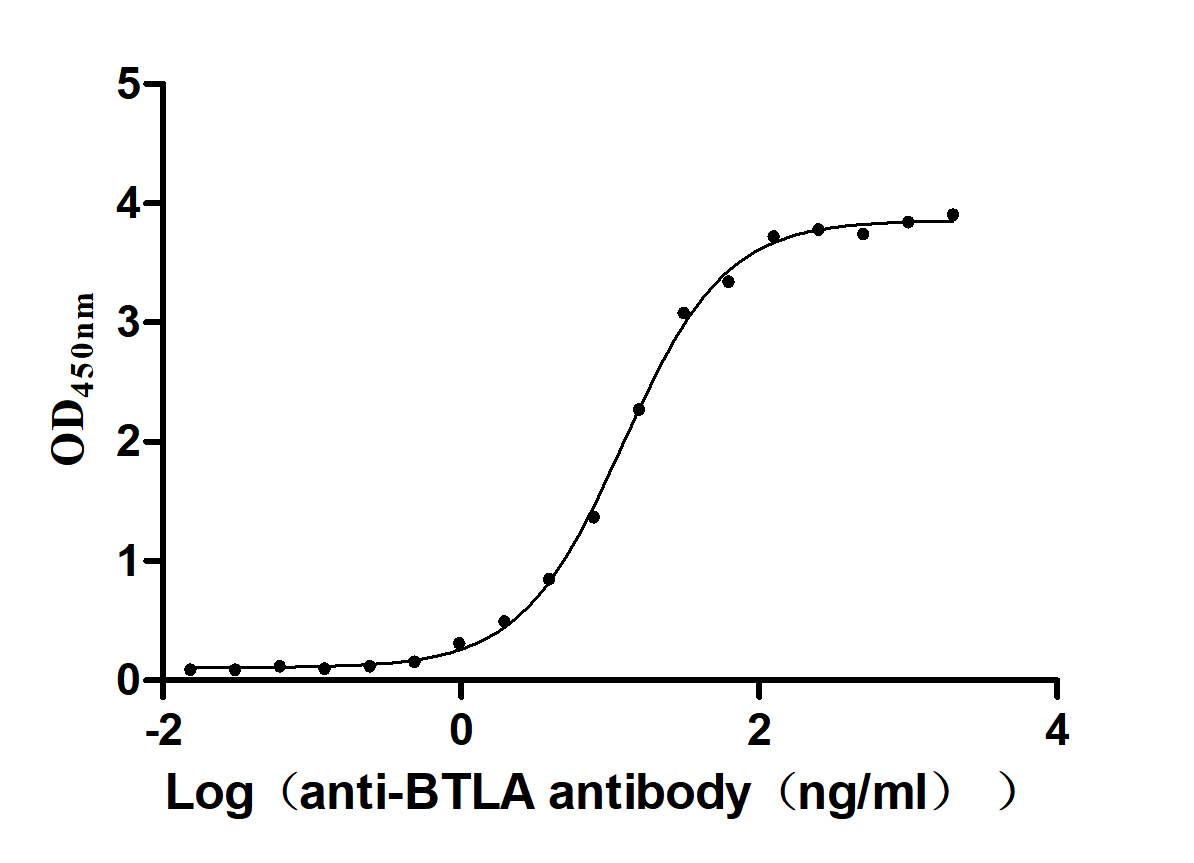



Comments
Leave a Comment