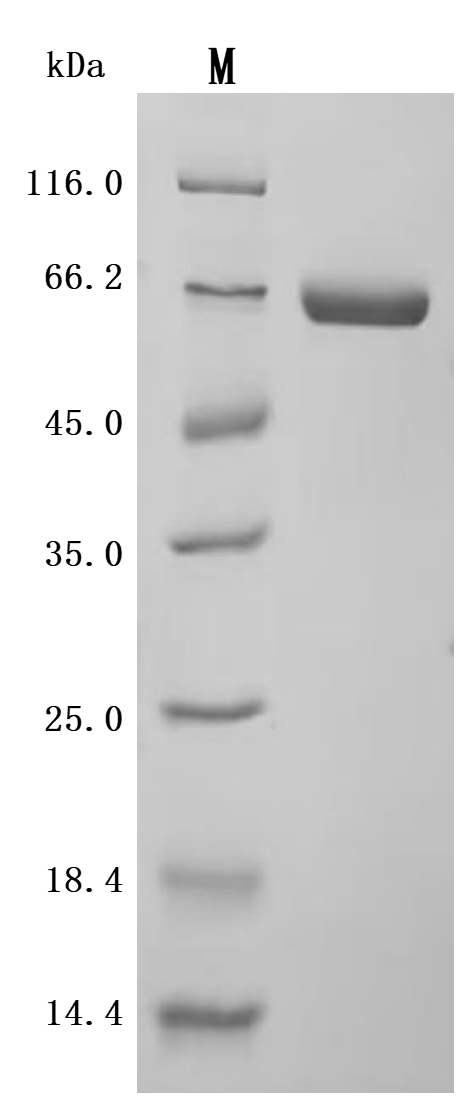
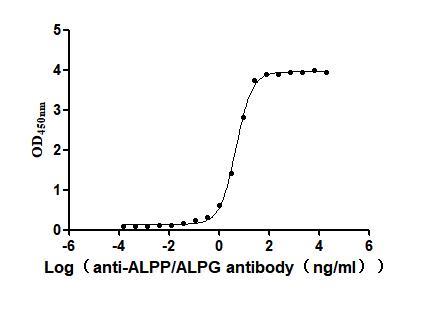
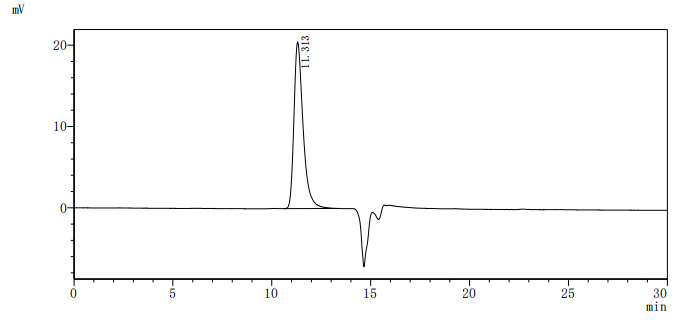
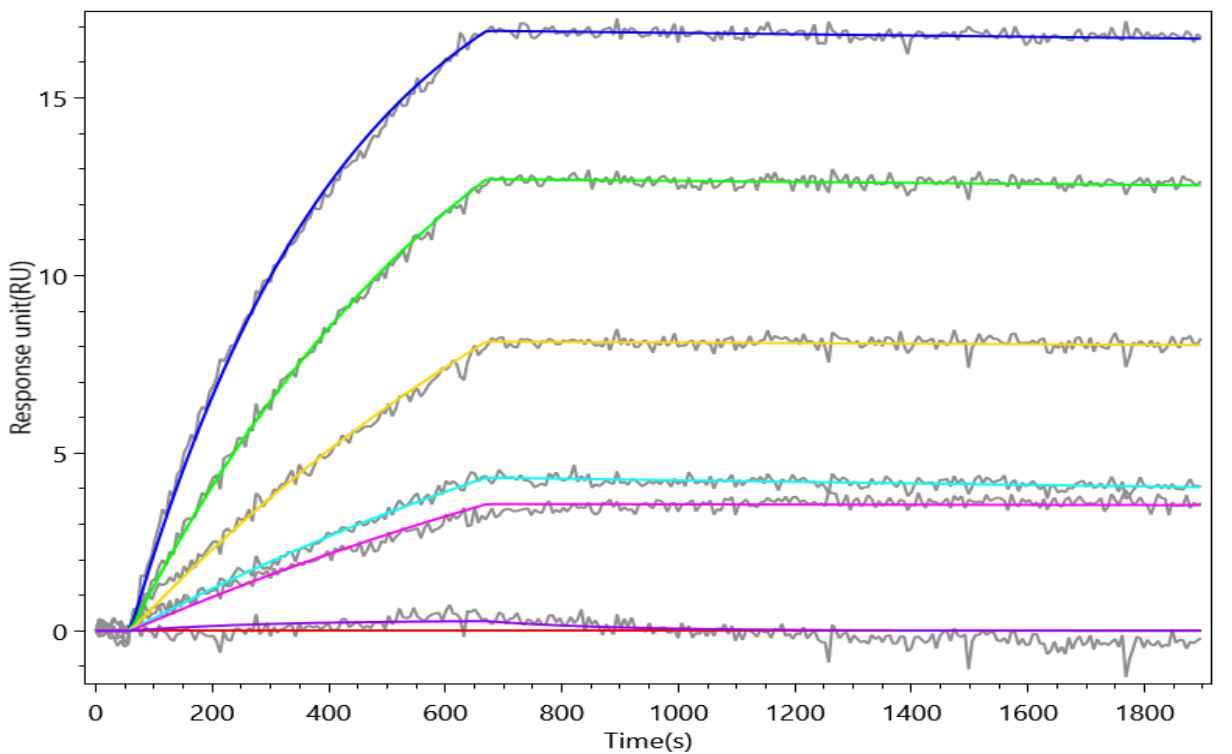
The recombinant human ALPP protein is a functionally active molecule synthesized in mammalian cells to ensure proper folding and native-like post-translational modifications. Covering amino acids 23 to 506 of the full-length mature ALPP sequence, it includes a C-terminal 10xHis tag for simplified purification and detection. This recombinant ALPP protein is provided as a lyophilized powder and meets high-quality standards, with purity greater than 95% confirmed by both SDS-PAGE and SEC-HPLC analyses. Functional validation via ELISA shows that when immobilized at 2 μg/mL, ALPP specifically binds to the anti-ALPP/ALPG recombinant antibody (CSB-RA001633MA1HU), with an EC50 ranging from 4.478 to 5.236 ng/mL. These properties make this recombinant ALPP a reliable reagent for applications in antibody screening, biomarker research, or enzyme activity studies.
Human ALPP serves critical functions in cellular transport and signaling. It is a glycoprotein that operates predominantly in the placenta during gestation and is crucial for various biological processes, including fetal development. ALPP is encoded by the ALPP gene located on chromosome 2q37.1 and is characterized by its dimeric structure, consisting of two subunits approximately 65 kDa in mass and composed of 535 amino acids [1][2].
ALPP mainly facilitates the hydrolysis of phosphate monoesters at alkaline pH levels [3]. This enzymatic activity is vital for various physiological processes, including the active transport of phosphates across cell membranes, which is essential for cellular energy metabolism and cell signaling [4]. The presence of ALPP in maternal serum can reflect placental health and developmental status, making it a significant biomarker in prenatal diagnostics [2]. Elevated levels of ALPP are often observed in conditions of placental insufficiency, indicating potential complications such as preeclampsia [5].
Furthermore, ALPP exhibits unique regulatory roles in cellular differentiation and migration. It is believed to guide the movement of cells during placentation and contribute to the immune modulation necessary for protecting the fetus from maternal immune responses [1][2]. Moreover, ALPP's ability to dephosphorylate various substrates, including matrix metalloproteinases (such as MMP-2), suggests its involvement in tissue remodeling and inflammation regulation [6][7]. Thus, ALPP not only plays a pivotal role in metabolic processes but also in extracellular matrix regulation and angiogenesis, which are vital for placental and fetal development [6][7].
References:
[1] V. Reiswich, N. Gorbokon, et al. Pattern of placental alkaline phosphatase (plap) expression in human tumors: a tissue microarray study on 12,381 tumors. The Journal of Pathology Clinical Research, vol. 7, no. 6, p. 577-589, 2021. https://doi.org/10.1002/cjp2.237
[2] U. Sharma, D. Pal, & R. Prasad. Alkaline phosphatase: an overview. Indian Journal of Clinical Biochemistry, vol. 29, no. 3, p. 269-278, 2013. https://doi.org/10.1007/s12291-013-0408-y
[3] P. Pickkers, S. Heemskerk, et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Critical Care, vol. 16, no. 1, 2012. https://doi.org/10.1186/cc11159
[4] S. Naik and P. Naik. A case report of incidentally elevated maternal serum alkaline phosphatase managed in a poor resource setting. International Journal of Reproduction Contraception Obstetrics and Gynecology, vol. 11, no. 9, p. 2586, 2022. https://doi.org/10.18203/2320-1770.ijrcog20222340
[5] S. Ruhi, J. Khan, et al. Evaluation of lipid profile, calcium and alkaline phosphatase in pregnancy induced hypertension women. Pharmaceutical and Biosciences Journal, p. 24-28, 2015. https://doi.org/10.20510/ukjpb/3/i2/89343
[6] M. Sarıahmetoğlu, B. Crawford, et al. Regulation of matrix metalloproteinase‐2 (mmp‐2) activity by phosphorylation. The Faseb Journal, vol. 21, no. 10, p. 2486-2495, 2007. https://doi.org/10.1096/fj.06-7938com
[7] M. Sarıahmetoğlu, M. Skrzypiec‐Spring, et al. Phosphorylation status of matrix metalloproteinase 2 in myocardial ischaemia–reperfusion injury. Heart, vol. 98, no. 8, p. 656-662, 2012. https://doi.org/10.1136/heartjnl-2011-301250