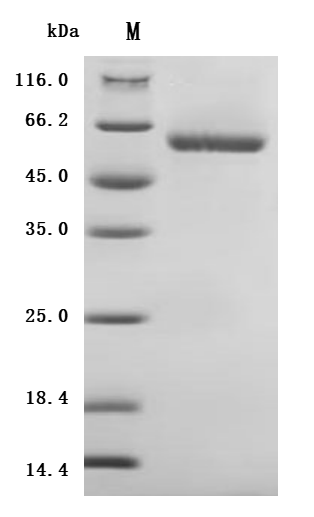
Recombinant human LAG3, spanning residues 23–434 of isoform 1, is expressed in mammalian cells and purified to >90% purity by SDS-PAGE. Also known as Lymphocyte activation gene 3 protein, LAG-3, CD223, LAG3, or FDC, this fragment (~46.1 kDa) carries a C-terminal 10×His tag for easy purification. It activity has been validated.
Potential Applications
(Note: The following applications are based on the known biological functions of this protein and scientific literature predictions. Our company has not validated all listed applications, and specific effects need to be verified by customers according to their experimental requirements. We recommend conducting small-scale preliminary experiments before formal studies.)
Mechanism Overview
LAG3 (Lymphocyte Activation Gene-3) is an important immune checkpoint receptor primarily expressed on activated T cells, regulatory T cells, and NK cells. By binding to MHC-II molecules, LAG3 negatively regulates T cell activation and proliferation, playing a key role in maintaining immune homeostasis and preventing excessive immune responses [1]. As an emerging immunotherapy target, LAG3 is pivotal in tumor immune evasion mechanisms [2].
Main Application Areas
1. Immune Checkpoint Functional Studies
Applications & Experimental Use:
This recombinant protein (purity >90%, endotoxin <1.0 EU/μg) can be utilized in in vitro binding experiments to investigate the interaction mechanism between LAG3 and its ligand MHC-II. Techniques such as ELISA and surface plasmon resonance (SPR) can be employed to measure binding affinity and kinetic parameters. The C-terminal 10×His tag facilitates both protein purification and detection, while expression in a mammalian cell system ensures proper folding and post-translational modifications.
Scientific Basis:
Studies indicate that LAG3 has a binding affinity for MHC-II molecules that is 100 times greater than that of the CD4 molecule, underpinning its key role in regulating T cell activation [3]. The extracellular domain of LAG3 specifically binds to the D1 domain of MHC-II, thereby inhibiting T cell receptor signaling [4].
2. Anti-LAG3 Antibody Drug Development and Screening
Applications & Experimental Use:
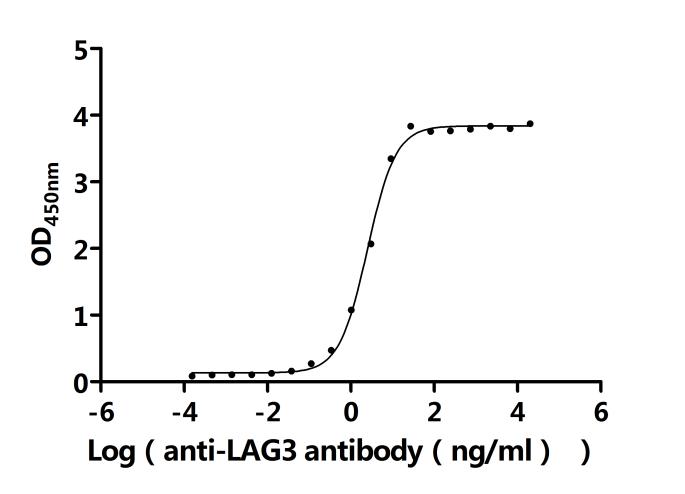
This recombinant protein can serve as an antigen for screening, characterizing, and quality controlling anti-LAG3 monoclonal antibodies. Competitive binding assays are used to evaluate antibody specificity, while flow cytometry or immunohistochemistry can verify antibody functionality. The product has been functionally validated by ELISA, with an EC50 value ranging from 2.306 to 2.731 ng/mL, ensuring experimental reliability and reproducibility.
Scientific Basis:
Anti-LAG3 antibodies, such as relatlimab, have received FDA approval for the treatment of melanoma, demonstrating promising clinical outcomes [5]. Multiple clinical trials have confirmed that the combination of LAG3 antibodies with PD-1 inhibitors can significantly enhance the efficacy of tumor immunotherapy [6].
3. Tumor Immune Microenvironment Studies
Applications & Experimental Use:
This protein is used to construct in vitro tumor immune microenvironment models to study the role of LAG3 in T cell exhaustion and tumor immune evasion. Co-culture experiments can monitor the effects of LAG3 on T cell proliferation, cytokine secretion, and cytotoxicity. Its high purity and low endotoxin levels ensure that experimental results remain free from confounding contaminants.
Scientific Basis:
LAG3 is highly expressed on tumor-infiltrating lymphocytes and is closely linked with the T cell exhaustion state [7]. Research shows that LAG3-positive TILs correlate with poor patient prognosis, making LAG3 an important biomarker in immunotherapy [8].
4. Mechanistic Studies of Immune Cell Function Regulation
Applications & Experimental Use:
This protein is employed to investigate the role of LAG3 in regulating the function of regulatory T cells (Tregs). By adding recombinant LAG3 protein, researchers can observe its effects on Treg-mediated suppression. Additionally, it can be used in T cell activation experiments to analyze the modulatory impact of LAG3 on T cell proliferation, differentiation, and effector functions. The use of a mammalian expression system ensures that the protein maintains its native conformation and bioactivity.
Scientific Basis:
LAG3 is constitutively expressed on CD4+CD25+ Treg cells and is crucial for their suppressive function [9]. Mice lacking LAG3 exhibit heightened T cell responses and a predisposition to autoimmune disorders [10].
5. Development of Biomarker Detection Methods
Applications & Experimental Use:
This protein can be used as a standard in developing quantitative detection methods for LAG3, including ELISA and electrochemiluminescence immunoassays. It is applicable for detecting soluble LAG3 (sLAG3) levels in serum, plasma, or tissue samples. The His tag aids in establishing standard curves and quality control, ensuring the accuracy and reproducibility of the detection methods.
Scientific Basis:
Serum levels of sLAG3 are associated with the severity and prognosis of various diseases, making it a potential diagnostic and monitoring biomarker. Studies have demonstrated that sLAG3 can serve as an effective indicator for evaluating the outcomes of immunotherapy [11].
References
[1] Triebel, F., Jitsukawa, S., Baixeras, E., Roman-Roman, S., Genevee, C., Viegas-Pequignot, E., & Hercend, T. (1990). LAG-3, a novel lymphocyte activation gene closely related to CD4. Journal of Experimental Medicine, 171(5), 1393-1405.
[2] Maruhashi, T., Okazaki, I. M., Sugiura, D., Takahashi, S., Maeda, T. K., Shimizu, K., & Okazaki, T. (2018). LAG-3 inhibits the activation of CD4+ T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nature Immunology, 19(12), 1415-1426.
[3] Huard, B., Prigent, P., Tournier, M., Bruniquel, D., & Triebel, F. (1995). CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. European Journal of Immunology, 25(9), 2718-2721.
[4] Baixeras, E., Huard, B., Miossec, C., Jitsukawa, S., Martin, M., Hercend, T., & Triebel, F. (1992). Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. Journal of Experimental Medicine, 176(2), 327-337.
[5] Tawbi, H. A., Schadendorf, D., Lipson, E. J., Ascierto, P. A., Matamala, L., Castillo Gutiérrez, E., ... & Long, G. V. (2022). Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. New England Journal of Medicine, 386(1), 24-34.
[6] Woo, S. R., Turnis, M. E., Goldberg, M. V., Bankoti, J., Selby, M., Nirschl, C. J., ... & Drake, C. G. (2012). Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Research, 72(4), 917-927.
[7] Shi, A. P., Tang, X. Y., Xiong, Y. L., Zheng, K. F., Liu, Y. J., Shi, X. G., Lv, Y., Jiang, T., Ma, N., & Zhao, J. B. (2022). Immune checkpoint LAG3 and its ligand FGL1 in cancer. Frontiers in Immunology, 12, 785091.
[8] Friedlaender, A., Tsantoulis, P., Chevallier, M., De Velasco, M. A., Addeo, A., & Tsoukalas, N. (2021). The multi-dimensional impact of LAG-3 on cancer immunotherapy. Cancer Letters, 518, 92-101.
[9] Liang, B., Workman, C., Lee, J., Chew, C., Dale, B. M., Colonna, L., ... & Vignali, D. A. (2008). Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. Journal of Immunology, 180(9), 5916-5926.
[10] Workman, C. J., Dugger, K. J., & Vignali, D. A. (2002). Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. Journal of Immunology, 169(10), 5392-5395.
[11] Solinas, C., Migliori, E., De Silva, P., & Willard-Gallo, K. (2019). LAG3: the biological processes that motivate targeting this immune checkpoint molecule in human cancer. Cancers, 11(8), 1213.