[1] Bai, Rui, and Cheng Yuan. "Kita-kyushu lung cancer antigen-1 (KK-LC-1): a promising cancer testis antigen." Aging and Disease 13.4 (2022): 1267.
[2] Hsu, Robert, et al. "Molecular characterization of Kita-Kyushu lung cancer antigen (KK-LC-1) expressing carcinomas." Oncotarget 12.25 (2021): 2449.
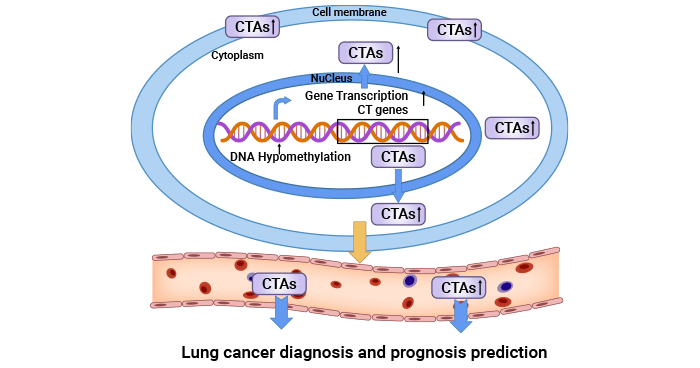
[3] Yang, Ping, et al. "Cancer/testis antigens as biomarker and target for the diagnosis, prognosis, and therapy of lung cancer." Frontiers in Oncology 12 (2022): 864159.
[4] Zhou, Xingchun, et al. "Heterogeneous expression of CT10, CT45 and GAGE7 antigens and their prognostic significance in human breast carcinoma." Japanese Journal of Clinical Oncology 43.3 (2013): 243-250.
[5] Chen, Zhiqiang, et al. "Hypomethylation‐mediated activation of cancer/testis antigen KK‐LC‐1 facilitates hepatocellular carcinoma progression through activating the Notch1/Hes1 signalling." Cell Proliferation 52.3 (2019): e12581.
[6] Norberg, Scott, et al. "A phase I trial of T-cell receptor gene therapy targeting KK-LC-1 for gastric, breast, cervical, lung and other KK-LC-1 positive epithelial cancers." (2022): TPS2678-TPS2678.
[7] Kang, Yanli, et al. "Cancer-testis antigen KK-LC-1 is a potential biomarker associated with immune cell infiltration in lung adenocarcinoma." BMC cancer 22.1 (2022): 834.
[8] Chen, Zhiqiang, et al. "Hypomethylation‐mediated activation of cancer/testis antigen KK‐LC‐1 facilitates hepatocellular carcinoma progression through activating the Notch1/Hes1 signalling." Cell Proliferation 52.3 (2019): e12581.
[9] Mabjeesh, Nicola J., and S. Amir. "Hypoxia-inducible factor (HIF) in human tumorigenesis." Histology and histopathology (2007).
[10] Marcinkowski, Bridget, et al. "Preclinical characterization of a KK-LC-1-specific T cell receptor for the treatment of epithelial cancers." Cancer Research 79.13_Supplement (2019): 1429-1429.
[11] Kim, Min Kyu, et al. "Clinical significance of HIF-2α immunostaining area in radioresistant cervical cancer." Journal of Gynecologic Oncology 22.1 (2011): 44.
[12] Gul, Samina, et al. "Stemness signature and targeted therapeutic drugs identification for Triple Negative Breast Cancer." Scientific Data 10.1 (2023): 815.
[13] Zeng, Yaoying, et al. "Integrating Network Pharmacology, Molecular Docking, and Experimental Validation to Investigate the Mechanism of (−)-Guaiol Against Lung Adenocarcinoma." Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 28 (2022): e937131-1.
[14] Chen, Chen, et al. "Multiomics analysis reveals CT83 is the most specific gene for triple negative breast cancer and its hypomethylation is oncogenic in breast cancer." Scientific reports 11.1 (2021): 12172.
[15] Gao, Junyi, et al. "Up‐regulation of CDHR5 expression promotes malignant phenotype of pancreatic ductal adenocarcinoma." Journal of Cellular and Molecular Medicine 24.21 (2020): 12726-12735.
[16] Inoue-Shibui, Aya, et al. "A novel deletion in the C-terminal region of HSPB8 in a family with rimmed vacuolar myopathy." Journal of Human Genetics 66.10 (2021): 965-972.
[17] Li, Qingyang, et al. "Natural high-avidity T-cell receptor efficiently mediates regression of cancer/testis antigen 83 positive common solid cancers." Journal for Immunotherapy of Cancer 10.7 (2022).
[18] Marcinkowski, Bridget, et al. "Cancer targeting by TCR gene-engineered T cells directed against Kita-Kyushu Lung Cancer Antigen-1." Journal for immunotherapy of cancer 7 (2019): 1-9.
[19] Lin, Min, et al. "Recent advances on the molecular mechanism of cervical carcinogenesis based on systems biology technologies." Computational and Structural Biotechnology Journal 17 (2019): 241-250.
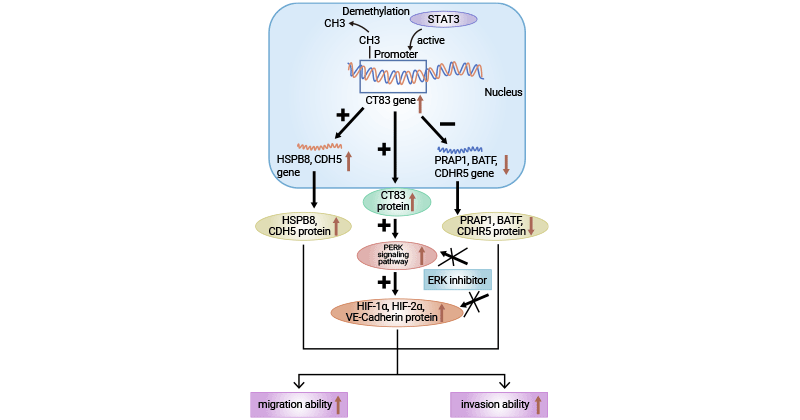
[20] Qiao Yingnan. The mechanisms underlying regulation of proto-oncogene CT83 expression and promotion of cervical cancer cell migration and invasion [D]. Soochow University, 2022.
[21] Chen, Chen, et al. "Multiomics analysis reveals CT83 is the most specific gene for triple negative breast cancer and its hypomethylation is oncogenic in breast cancer." Scientific reports 11.1 (2021): 12172.
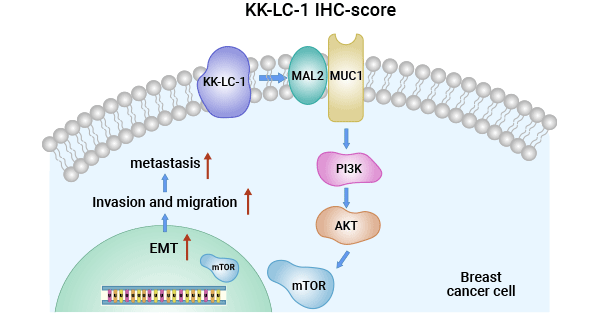
[22] Zhu, Xudong, et al. "Targeting KK-LC-1 inhibits malignant biological behaviors of triple-negative breast cancer." Journal of Translational Medicine 21.1 (2023): 184.
[23] Ichiki, Yoshinobu, et al. "Development of adoptive immunotherapy with KK‐LC‐1‐specific TCR‐transduced γδT cells against lung cancer cells." Cancer Science 111.11 (2020): 4021-4030.
[24] Hu, Yeting, et al. "Quantitative Analysis on Molecular Characteristics Evolution of Gastric Cancer Progression and Prognosis." Advanced Biology 7.10 (2023): 2300129.
[25] Chen, Zhiqiang, et al. "Hypomethylation‐mediated activation of cancer/testis antigen KK‐LC‐1 facilitates hepatocellular carcinoma progression through activating the Notch1/Hes1 signalling." Cell Proliferation 52.3 (2019): e12581.
[26] Otsuka, Toshikazu, et al. "Detection of Kita-Kyushu Lung Cancer Antigen-1, a Cancer/Testis Antigen, in the Stomach Close to a Cancerous Condition." Journal of Cancer 13.14 (2022): 3526.
[27] Fukuyama, Takashi, et al. "Expression of KK-LC-1, a cancer/testis antigen, at non-tumour sites of the stomach carrying a tumour." Scientific reports 8.1 (2018): 6131.
[28] Bai, Rui, and Cheng Yuan. "Kita-kyushu lung cancer antigen-1 (KK-LC-1): a promising cancer testis antigen." Aging and Disease 13.4 (2022): 1267.
[29] Marcinkowski, Bridget, et al. "Cancer targeting by TCR gene-engineered T cells directed against Kita-Kyushu Lung Cancer Antigen-1." Journal for immunotherapy of cancer 7 (2019): 1-9.
[30] Scheifele, Fabian, et al. "Abstract LB442: Novel antibodies against a KK-LC-1-derived peptide presented on HLA-A* 01 on tumor cells." Cancer Research 84.7_Supplement (2024): LB442-LB442.


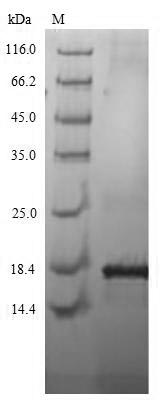
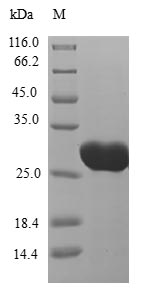
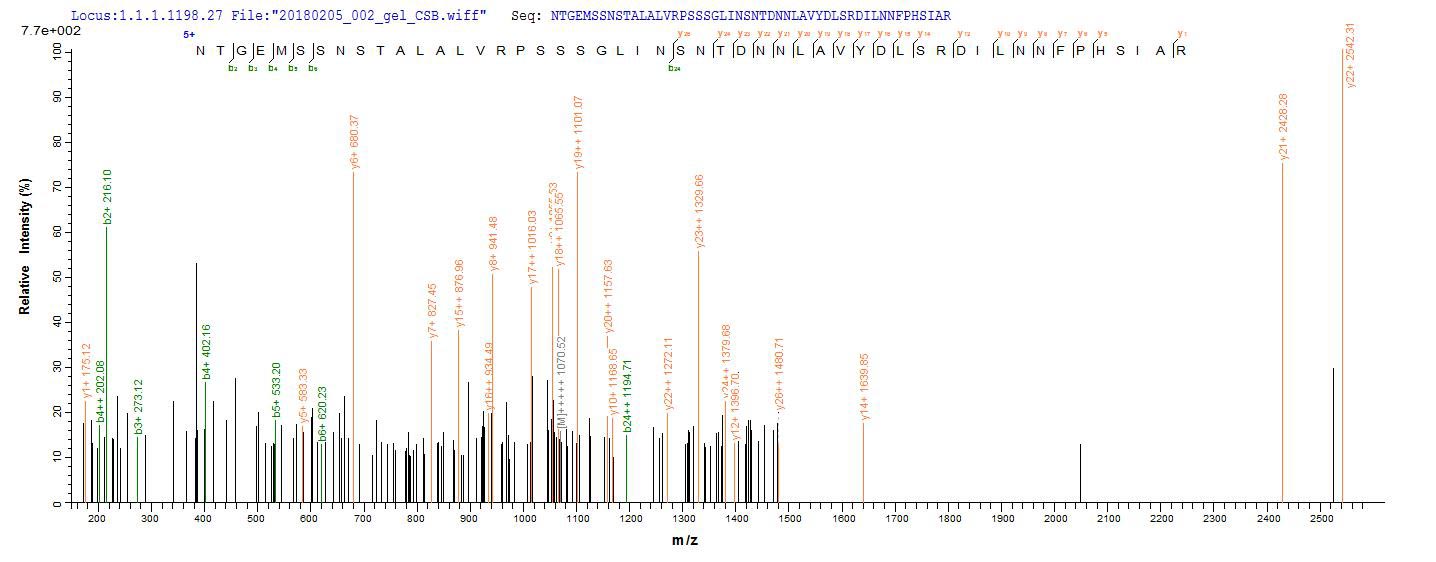


Comments
Leave a Comment