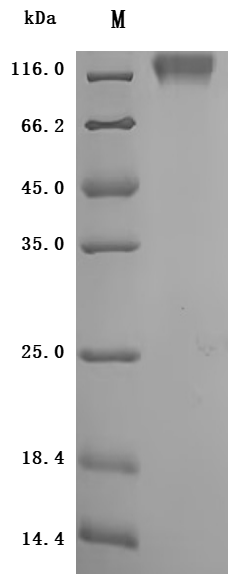
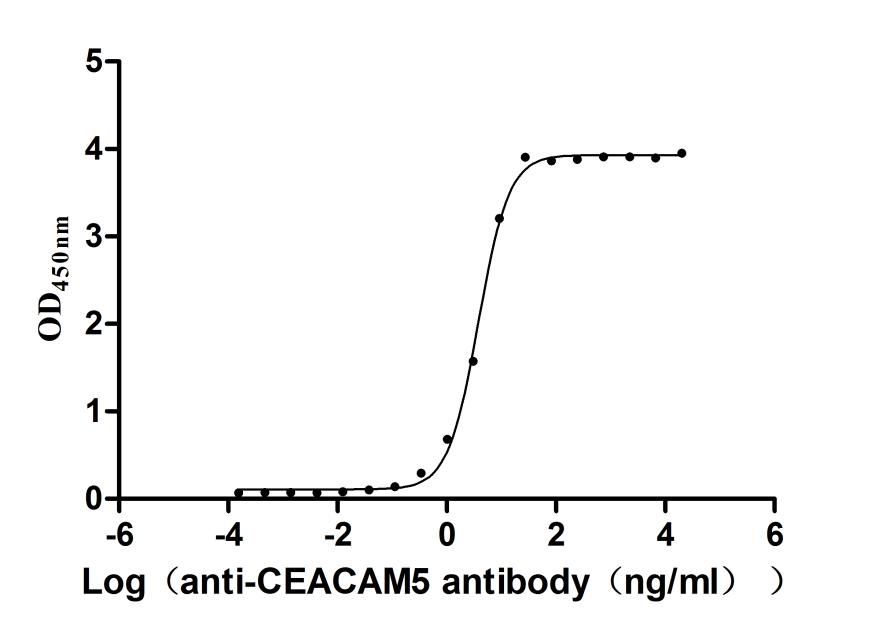
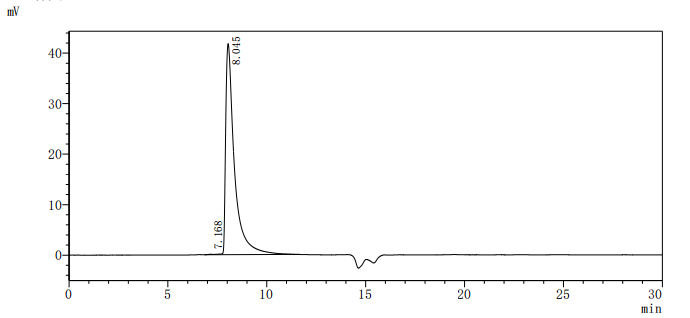
The recombinant CEACAM5 protein from Macaca fascicularis is a biologically active molecule produced in a mammalian expression system, encompassing the amino acids 1–685 of the native CEACAM5 to ensure native-like structure and function. It includes a C-terminal 10xHis tag for ease of purification and detection. This recombinant CEACAM5 protein is provided as a lyophilized powder and demonstrates exceptional purity—over 95%, as confirmed by both SDS-PAGE and SEC-HPLC analyses. Endotoxin content is rigorously controlled and remains below 1.0 EU/µg, as tested by the LAL method. Functional validation was conducted using ELISA, where the CEACAM5 protein, immobilized at 2 μg/mL, binds specifically to the anti-CEACAM5 recombinant antibody (CSB-RA005165MA4HU), yielding an EC50 between 3.572 and 4.044 ng/mL. These features make it highly reliable for use in immunological and biochemical assays involving CEACAM5.
The CEACAM5 protein plays a significant role in various biological processes, including cell adhesion, signaling, and immune responses in primates such as the rhesus macaque (Macaca mulatta). As a member of the CEACAM family, CEACAM5 is implicated in tumor progression and metastasis, particularly in epithelial tissues, where it can modulate cellular interactions and influence cell motility [1][2].
In the context of Macaca mulatta, CEACAM5 is involved in the pathophysiology of certain diseases, including cancers. The protein's expression can be upregulated in various carcinomas, and its interaction with other proteins can facilitate tumor cell adhesion and extravasation [3][4]. This is particularly relevant considering that the rhesus macaque model is frequently employed in biomedical research to study human diseases, including cancer, due to its close genetic and physiological similarities to humans [5][6].
Moreover, CEACAM5 has relevance concerning the immune response, as it may influence T-cell mediated immunity. The regulation of CEACAM5 can alter the immune landscape in tumors, affecting both tumor immunogenicity and the efficacy of therapeutic interventions [7][8]. The CEACAM5 protein's role as a potential biomarker for diagnosis and treatment response in cancers in macaque models can also provide insights into human health and disease management, highlighting its importance in translational medicine research [9][10].
Additionally, research utilizing bioinformatics and genomic analyses has contributed to our understanding of CEACAM5's structure and function. Recently, studies have applied high-throughput sequencing methods to profile CEACAM5 and related gene expressions across various tissues [11][12][4], suggesting a functional diversity that reflects evolutionary adaptations among primates, including Macaca mulatta.
References:
[1] M. Lopez‐Cruzan, N. Walter, et al. Phosphatidylethanol in whole blood of rhesus monkeys correlates with ethanol consumption. Alcoholism Clinical and Experimental Research, vol. 45, no. 4, p. 689-696, 2021. https://doi.org/10.1111/acer.14584
[2] L. Fu, X. Li, et al. A comprehensive profiling of t- and b-lymphocyte receptor repertoires from a chinese-origin rhesus macaque by high-throughput sequencing. Plos One, vol. 12, no. 8, p. e0182733, 2017. https://doi.org/10.1371/journal.pone.0182733
[3] H. Yang, F. Ito, et al. Understanding the structural basis of hiv-1 restriction by the full length double-domain apobec3g. Nature Communications, vol. 11, no. 1, 2020. https://doi.org/10.1038/s41467-020-14377-y
[4] J. Li, Z. Fan, et al. Genomic copy number variation study of nine macaca species provides new insights into their genetic divergence, adaptation, and biomedical application. Genome Biology and Evolution, vol. 12, no. 12, p. 2211-2230, 2020. https://doi.org/10.1093/gbe/evaa200
[5] Ń. Otting, C. Heijmans, M. Wiel, N. Groot, G. Doxiadis, & R. Bontrop. A snapshot of the mamu-b genes and their allelic repertoire in rhesus macaques of chinese origin. Immunogenetics, vol. 60, no. 9, p. 507-514, 2008. https://doi.org/10.1007/s00251-008-0311-5
[6] A. Zimin, A. Cornish, et al. A new rhesus macaque assembly and annotation for next-generation sequencing analyses. Biology Direct, vol. 9, no. 1, 2014. https://doi.org/10.1186/1745-6150-9-20
[7] S. Handley, C. Desai, et al. Siv infection-mediated changes in gastrointestinal bacterial microbiome and virome are associated with immunodeficiency and prevented by vaccination. Cell Host & Microbe, vol. 19, no. 3, p. 323-335, 2016. https://doi.org/10.1016/j.chom.2016.02.010
[8] J. Satkoski, R. Malhi, S. Kanthaswamy, R. Tito, V. Malladi, & D. Smith. Pyrosequencing as a method for snp identification in the rhesus macaque (macaca mulatta). BMC Genomics, vol. 9, no. 1, p. 256, 2008. https://doi.org/10.1186/1471-2164-9-256
[9] J. Almeida, A. Pinho, J. Oliveira, O. Fajarda, & D. Pratas. Gto: a toolkit to unify pipelines in genomic and proteomic research. Softwarex, vol. 12, p. 100535, 2020. https://doi.org/10.1016/j.softx.2020.100535
[10] W. Nix, B. Jiang, K. Maher, E. Strobert, & M. Oberste. Identification of enteroviruses in naturally infected captive primates. Journal of Clinical Microbiology, vol. 46, no. 9, p. 2874-2878, 2008. https://doi.org/10.1128/jcm.00074-08
[11] Z. Su, J. Zhang, et al. Species specific exome probes reveal new insights in positively selected genes in nonhuman primates. Scientific Reports, vol. 6, no. 1, 2016. https://doi.org/10.1038/srep33876
[12] P. Najarro, H. Lee, J. Fox, J. Pease, & G. Smith. Yaba-like disease virus protein 7l is a cell-surface receptor for chemokine ccl1. Journal of General Virology, vol. 84, no. 12, p. 3325-3336, 2003. https://doi.org/10.1099/vir.0.19591-0