ASCO Annual Meeting, as a wind vane in the field of oncology, attracts global attention every year. 2024 ASCO Annual Meeting is especially notable for the clinical progress reports of ADC drugs. From CLDN18.2 to HER2 to TROP2, the research progress of these star targets reflects the competitive landscape of global drug companies in this field. With the deepening of clinical trials and research on these drugs, the future may bring more breakthroughs and options for cancer treatment.
Let's take a look at some of the targets that made a splash at the ASCO annual meeting.
CLDN18.2
CLDN18.2 is a tight junction protein, a member of the Claudin family. It is expressed in normal gastric mucosal cells, but its expression is significantly up-regulated in tumor cells, especially in digestive system tumors such as gastric and pancreatic cancers. Due to its specific expression in tumor tissues, CLDN18.2 has become a promising therapeutic target, especially in the development of targeted therapeutic agents against gastric cancer.
Read more: Claudin 18.2, a “dark horse” target for anti-tumor therapy
At ASCO 2024, significant progress was made in ADC studies targeting CLDN18.2. LaNova Medicines' LM-302 showed positive Phase I/II clinical trial results in patients with advanced gastric or gastroesophageal junction cancer. Innovent Biologics' IBI343 demonstrated a manageable safety profile and encouraging efficacy in patients with advanced pancreatic ductal adenocarcinoma or biliary tract cancer, and has been recognized by the NMPA as a breakthrough therapy due to its "bystander effect". The ADC drug AZD0901 (CMG901), co-developed by AstraZeneca, has shown promising anti-tumor activity and a manageable safety profile in patients with advanced treated gastric cancer, achieving a confirmed ORR of 48% in the 2.2mg/kg dose group and a median OS of up to 11.8 months. Deciphera Pharmaceuticals' ATG-022 also exhibited preliminary antitumor activity and good tolerability in patients with advanced solid tumors.
Link to the meeting report:
LM-302:https://meetings.asco.org/abstracts-presentations/234096
IBI343:https://meetings.asco.org/abstracts-presentations/234144/
AZD0901:https://meetings.asco.org/abstracts-presentations/238349/
ATG-022:https://meetings.asco.org/abstracts-presentations/234108/
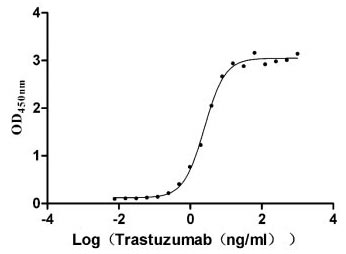
CUSABIO CLDN18.2 Recombinant Protein
Recombinant Human Claudin-18.2 (CLDN18.2)-VLPs (Active) (CSB-MP005498HU(A5))
HER2
HER2, formally known as the human epidermal growth factor receptor 2 (ERBB2), is a pivotal protein intricately involved in cellular proliferation and viability. Its overexpression in breast cancer is a significant indicator of tumor aggressiveness and is linked to an unfavorable prognosis. This makes HER2 a prime therapeutic target. Therapies targeting HER2 have revolutionized the treatment landscape, with monoclonal antibodies like trastuzumab (Herceptin) and antibody-drug conjugates (ADCs), such as Enhertu, demonstrating remarkable efficacy against HER2-positive breast and gastric adenocarcinomas. These advancements have not only enhanced patient outcomes but also underscore the significant strides made in oncology therapeutics.
Read more: The Oncogene HER2: a New Target for Drug-Resistant Tumors
At ASCO 2024, significant progress was made in the development of HER2-targeted ADC drugs, particularly Enhertu (DS-8201, detrastuzumab), co-developed by AstraZeneca and Daiichi Sankyo, which has been shown to perform well in the treatment of breast cancer. Preliminary results from the DESTINY-Breast06 trial showed that Enhertu met the PFS endpoint in HR+/HER2 low-expressing breast cancer met the PFS endpoint in Phase III and is expected to become a new standard of care. In addition, Hengrui Medicine's SHR-A1811 has also demonstrated potential in breast cancer treatment, and these results highlight the importance of HER2 targets in ADC drug development.
Link to the meeting report:
DESTINY-Breast06:https://meetings.asco.org/abstracts-presentations/238015
SHR-A1811:https://meetings.asco.org/abstracts-presentations/235117
CUSABIO HER2 Recombinant Protein
Recombinant Human Receptor tyrosine-protein kinase erbB-2 (ERBB2), partial (Active) (CSB-MP007763HU)
TROP2
TROP2 is a transmembrane glycoprotein that is highly expressed in a variety of tumors and is closely associated with tumor cell growth, proliferation and metastasis. It has been found in a variety of epithelial tumors, including prostate, esophageal squamous cell, ovarian, breast, colorectal, gastric, pancreatic and lung cancers. Due to its key role in tumor development, TROP2 has become an important target for anti-cancer drug research, especially in the development of antibody-drug couplings (ADCs), which shows great potential.
Read more: TROP2, An Important Target for Cancer Research
The field of ADC drug development for TROP2 targets presented mixed results at the 2024 ASCO Annual Meeting. DS-1062, developed in collaboration with AstraZeneca and Daiichi Sankyo, showed clinically meaningful OS improvement in a subgroup of non-squamous non-small cell lung cancer (NSCLC) patients, but failed to reach statistical significance in the overall trial population, resulting in its primary endpoint not being met. On the other hand, ColumboTech announced that its TROP2 ADC drug SKB264 in combination with KL-A167 showed excellent data in a Phase 2 study for the first-line treatment of advanced NSCLC, providing new hope for TROP2-targeted therapies in the field of NSCLC. Despite some positive progress, the issues of target concentration and risk of involution have also raised concerns, suggesting that future ADC drug development needs to look for new strategies and targets to improve success rates.
Link to the meeting report:
DS-1062:https://meetings.asco.org/abstracts-presentations/232868/
SKB264:https://meetings.asco.org/abstracts-presentations/239101/
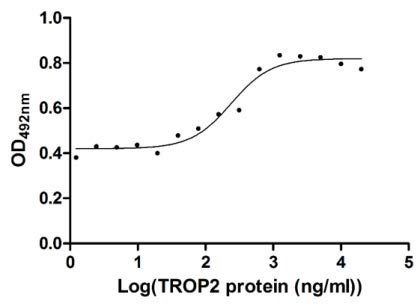
CUSABIO TROP2 Recombinant Protein
Recombinant Human Tumor-associated calcium signal transducer 2 (TACSTD2), partial (Active) (CSB-MP023072HU1)
CEACAM5
CEACAM5 is a member of the carcinoembryonic antigen-associated cell adhesion molecules (CEACAMs) family, which is highly expressed on the surface of a variety of epithelial tumor cells, including colorectal, gastric, pancreatic, lung adenocarcinomas, small-cell lung, bladder, and ovarian cancers.
Read more: CEACAM5: A Promising Member of CEACAM Family, A Potential Target for ADC Drug Research!
Merck presented first-in-human dosing results for its CEACAM5 ADC drug M9140 at the 2024 ASCO Annual Meeting. This suggests that ADC drugs against CEACAM5 targets are in the clinical development stage and are being evaluated for their potential and safety in the treatment of relevant cancers. Although specific clinical data and study results were not disclosed in detail, this development demonstrates the importance and development activity of CEACAM5 as an ADC drug target.
Link to the meeting report:
M9104:https://meetings.asco.org/abstracts-presentations/231480
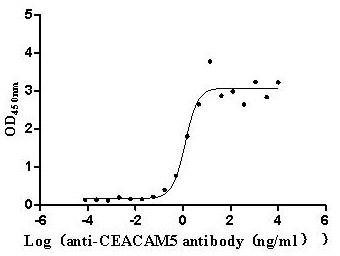
CUSABIO CEACAM5 Recombinant Protein
Recombinant Human Carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) (E398K) (Active) (CSB-MP005165HU)
At the 2024 ASCO Annual Meeting, several companies presented their clinical advances in the field of ADCs. In addition to those mentioned above, positive results in multiple tumor types with MyviBio's NECTIN-4 ADC 9MW2821, significant results in pancreatic cancer with Leprechaun Bio's TF ADC MRG004A and nasopharyngeal carcinoma with its EGFR ADC MRG003 in the backline of nasopharyngeal carcinoma. Henson Pharmaceuticals' B7H3 ADC HS-20093 data in SLCC backline treatment, Biotek's FRα ADC BAT8006's excellent performance in ovarian cancer, and Rongchang Biotech's MSLN ADC RC88's clinical progress in ovarian cancer, NSCLC, and cervical cancer.
These results highlight the innovation and potential of ADC drugs in the field of tumor therapy. With further research and development of these drugs, we have reason to believe that ADC drugs will bring more effective treatment options for patients, and the future is promising.
With years of experience in protein, antibody, and ELISA kit development, CUSABIO can tailor products, services, and solutions to meet customer needs at various stages of ADC drug development.
Antibody Discovery and Optimization
-
Anti-payload Antibody Customization Service
DAR Detection, PK
Drug Development Solutions
CUSABIO team. New Power in the Fight Against Cancer: Spotlight on Rising Stars of ADC Drugs at the 2024 ASCO Annual Meeting. https://www.cusabio.com/c-21183.html

-AC1.jpg)





Comments
Leave a Comment