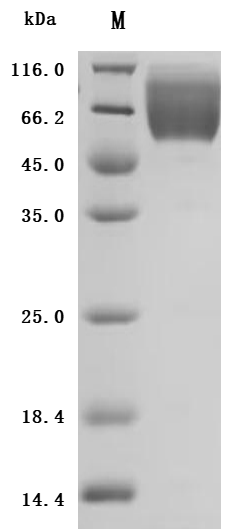
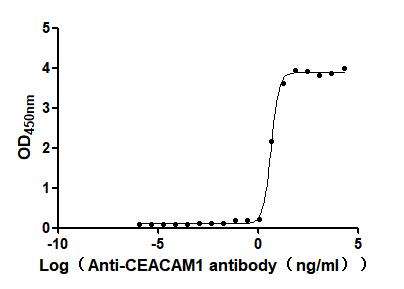
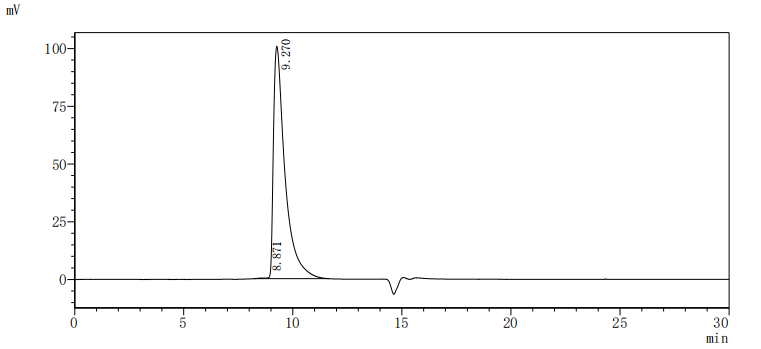
This recombinant human CEACAM1, encompassing residues 35-428 of the native CEACAM1 protein, is expressed in mammalian cells with a C-terminal 10×His-Avi tag and site-specific biotinylation. The recombinant CEACAM1 protein exhibits exceptional purity (>95% by SDS-PAGE) and low endotoxin content (<1.0 EU/μg, LAL method). Its activity has been tested through functional ELISA, demonstrating high-affinity binding to anti-CEACAM1 recombinant antibody(CSB-RA005157MA1HU), with the EC50 of 3.432–6.079 ng/mL. The Avi tag enables controlled biotinylation for streptavidin-based assays (e.g., flow cytometry, SPR), while the His tag facilitates purification without disrupting functional domains. This biotinylated CEACAM1 is optimized for studying homophilic/heterophilic interactions, immune checkpoint mechanisms, and therapeutic target validation in oncology and inflammatory disease research.
CEACAM1 is a member of the carcinoembryonic antigen (CEA) gene family that plays multifaceted roles in human biology, particularly in cell adhesion, immune response modulation, and regulation of metabolic processes. Evidence suggests that CEACAM1 functions as a signaling receptor that impacts various pathways associated with immune responses and cellular behavior.
One key function of CEACAM1 is its involvement in immune modulation. In human neutrophils, CEACAM1 is known to negatively regulate interleukin-1 beta production in response to lipopolysaccharide (LPS) activation by recruiting SHP-1 to the SYK-TLR4-CEACAM1 complex, thereby limiting inflammatory responses [1]. Additionally, CEACAM1 exhibits co-inhibitory functions in T cells, affecting both T cell receptor (TCR) signaling and the interaction with other receptors like CTLA-4, operating similarly to inhibitory receptors [2]. Moreover, its expression in CD4+ T cells is observed in conditions such as neonatal sepsis, highlighting its role in immune suppression during such pathological states [3].
CEACAM1 is also integral to vascular physiology, including angiogenesis and endothelial functions. For instance, it promotes endothelial cell migration and sprouting in cooperation with vascular endothelial growth factor (VEGF), enhancing angiogenesis [4]. Deficiency in CEACAM1 has been linked with vascular alterations and disrupted endothelial functions, as shown in studies revealing increased signaling through VEGFR-2 when CEACAM1 is absent [5], [6].
In addition to its roles in immune and vascular systems, CEACAM1 is critically involved in regulating metabolic processes, particularly in the liver. It has been demonstrated that reduced hepatic CEACAM1 levels contribute to insulin resistance and metabolic disorders like non-alcoholic fatty liver disease (NAFLD) [7], [8]. Its involvement in lipid storage regulation has been articulated through interactions with fatty acid transport mechanisms, emphasizing its significance in energy metabolism [9]. CEACAM1's phosphorylation status also determines its functional outcomes, with implications for downstream signaling pathways critical in insulin signaling and lipid metabolism [10].
Furthermore, CEACAM1 has been implicated in tumor biology, where it can function as a tumor suppressor and contribute to processes such as cellular senescence in response to DNA damage, particularly through p53 regulation [11]. Changes in CEACAM1 expression and isoform ratios are noted in various cancers; for example, its overexpression in the context of melanoma progression points to a dual role in promoting tumor growth and immune evasion [12].
References:
[1] R. Lu, H. Pan, & J. Shively. Ceacam1 negatively regulates il-1β production in lps activated neutrophils by recruiting shp-1 to a syk-tlr4-ceacam1 complex. Plos Pathogens, vol. 8, no. 4, p. e1002597, 2012. https://doi.org/10.1371/journal.ppat.1002597
[2] L. Chen, Z. Chen, et al. The short isoform of the ceacam1 receptor in intestinal t cells regulates mucosal immunity and homeostasis via tfh cell induction. Immunity, vol. 37, no. 5, p. 930-946, 2012. https://doi.org/10.1016/j.immuni.2012.07.016
[3] M. Flier, D. Sharma, et al. Increased cd4+ t cell co-inhibitory immune receptor ceacam1 in neonatal sepsis and soluble-ceacam1 in meningococcal sepsis: a role in sepsis-associated immune suppression? Plos One, vol. 8, no. 7, p. e68294, 2013. https://doi.org/10.1371/journal.pone.0068294
[4] J. MacManiman, A. Meuser, et al. Human cytomegalovirus-encoded pul7 is a novel ceacam1-like molecule responsible for promotion of angiogenesis. Mbio, vol. 5, no. 6, 2014. https://doi.org/10.1128/mbio.02035-14
[5] Y. Zhang, Y. Wang, et al. Elevation of neutrophil carcinoembryonic antigen‐related cell adhesion molecule 1 associated with multiple inflammatory mediators was related to different clinical stages in ischemic stroke patients. Journal of Clinical Laboratory Analysis, vol. 36, no. 7, 2022. https://doi.org/10.1002/jcla.24526
[6] S. Najjar, K. Ledford, et al. ceacam1 deletion causes vascular alterations in large vessels. Ajp Endocrinology and Metabolism, vol. 305, no. 4, p. E519-E529, 2013. https://doi.org/10.1152/ajpendo.00266.2013
[7] Q. Al–Share, A. DeAngelis, et al. Forced hepatic overexpression of ceacam1 curtails diet-induced insulin resistance. Diabetes, vol. 64, no. 8, p. 2780-2790, 2015. https://doi.org/10.2337/db14-1772
[8] S. Najjar. Mice with null mutation of ceacam1 develop nonalcoholic steatohepatitis. Hepatic Medicine Evidence and Research, p. 69, 2010. https://doi.org/10.2147/hmer.s8902
[9] J. Chean, C. Chen, et al. Human ceacam1-lf regulates lipid storage in hepg2 cells via fatty acid transporter cd36. Journal of Biological Chemistry, vol. 297, no. 5, p. 101311, 2021. https://doi.org/10.1016/j.jbc.2021.101311
[10] S. Zaidi, S. Asalla, et al. Loss of ceacam1 in hepatocytes causes hepatic fibrosis. European Journal of Clinical Investigation, vol. 54, no. 7, 2024. https://doi.org/10.1111/eci.14177
[11] S. Ap, R. Buser, et al. The ceacam1 tumor suppressor is an atm and p53-regulated gene required for the induction of cellular senescence by dna damage. Oncogenesis, vol. 1, no. 4, p. e7-e7, 2012. https://doi.org/10.1038/oncsis.2012.7
[12] I. Kube‐Golovin, M. Lyndіn, M. Wiesehöfer, & G. Wennemuth. Ceacam expression in an in-vitro prostatitis model. Frontiers in Immunology, vol. 14, 2023. https://doi.org/10.3389/fimmu.2023.1236343