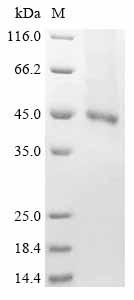
The partial human FLT3 protein-encoding gene (564-958aa) is fused with a 6xHis tag gene at the C-terminus to form the target gene. This target gene is amplified by PCR and inserted into expression vectors to create recombinant plasmids. These plasmids are transfected into Bacmids, which are transformed into E. coli cells for amplification and screening. The positive Bacmids containing the target gene are transfected into insect cells. After transfection, the cells will begin to produce recombinant baculovirus particles. The collected recombinant baculovirus particles are used to infect new insect cells to promote the expression of the FLT3 protein. The infected cells are cultured under appropriate temperature and culture conditions and then lysed to release the expressed recombinant FLT3 protein. The harvested recombinant human FLT3 protein is purified using affinity chromatography, with a purity of over 85% as confirmed by SDS-PAGE analysis.
Human FLT3 is a receptor tyrosine kinase that plays a critical role in hematopoiesis, particularly in the proliferation and differentiation of hematopoietic stem and progenitor cells. It is primarily expressed on early hematopoietic progenitors, including CD34+ cells, and is involved in various signaling pathways that regulate cell survival, growth, and differentiation [1][2][3]. The FLT3 gene is notable for its mutations, particularly in acute myeloid leukemia (AML), where internal tandem duplications (ITDs) and point mutations lead to constitutive activation of the receptor, contributing to leukemogenesis [4][5][6].
FLT3 binding to its ligand FLT3-L activates downstream signaling cascades, including the PI3K/AKT and MAPK pathways [7][8], which is crucial for the maintenance of hematopoietic stem cell (HSC) quiescence and homeostasis, as well as for the differentiation of dendritic cells [9][10]. FLT3 signaling is also important in the pathogenesis of hematological malignancies. Mutations in FLT3 can lead to aberrant signaling that promotes uncontrolled cell proliferation and survival, thereby contributing to the aggressive nature of AML [11][5][6].
References:
[1] T. Kindler, D. Lipka, & T. Fischer, Flt3 as a therapeutic target in aml: still challenging after all these years, Blood, vol. 116, no. 24, p. 5089-5102, 2010. https://doi.org/10.1182/blood-2010-04-261867
[2] E. Pulte, K. Norsworthy, Y. Wang, Q. Xu, H. Qosa, R. Gudi, et al., Fda approval summary: gilteritinib for relapsed or refractory acute myeloid leukemia with a flt3 mutation, Clinical Cancer Research, vol. 27, no. 13, p. 3515-3521, 2021. https://doi.org/10.1158/1078-0432.ccr-20-4271
[3] J. Zhang, O. Vakhrusheva, S. Bandi, Ö. Demirel, J. Kazi, R. Fernandes, et al., The phosphatases sts1 and sts2 regulate hematopoietic stem and progenitor cell fitness, Stem Cell Reports, vol. 5, no. 4, p. 633-646, 2015. https://doi.org/10.1016/j.stemcr.2015.08.006
[4] F. Abu-Duhier, A. Goodeve, W. Ga, C. Rs, P. Ir, & R. Jt, Identification of novel flt‐3 asp835 mutations in adult acute myeloid leukaemia, British Journal of Haematology, vol. 113, no. 4, p. 983-988, 2001. https://doi.org/10.1046/j.1365-2141.2001.02850.x
[5] J. Fan, L. Li, D. Small, & F. Rassool, Cells expressing flt3/itd mutations exhibit elevated repair errors generated through alternative nhej pathways: implications for genomic instability and therapy, Blood, vol. 116, no. 24, p. 5298-5305, 2010. https://doi.org/10.1182/blood-2010-03-272591
[6] A. Sallmyr, J. Fan, K. Datta, K. Kim, D. Grosu, P. Shapiro, et al., Internal tandem duplication of flt3 (flt3/itd) induces increased ros production, dna damage, and misrepair: implications for poor prognosis in aml, Blood, vol. 111, no. 6, p. 3173-3182, 2008. https://doi.org/10.1182/blood-2007-05-092510
[7] K. Verstraete, G. Vandriessche, M. Januar, J. Elegheert, A. Shkumatov, A. Desfosses, et al., Structural insights into the extracellular assembly of the hematopoietic flt3 signaling complex, Blood, vol. 118, no. 1, p. 60-68, 2011. https://doi.org/10.1182/blood-2011-01-329532
[8] D. Lin, T. Yin, M. Koren‐Michowitz, L. Ding, S. Gueller, S. Gery, et al., Adaptor protein lnk binds to and inhibits normal and leukemic flt3, Blood, vol. 120, no. 16, p. 3310-3317, 2012. https://doi.org/10.1182/blood-2011-10-388611
[9] C. Eidenschenk, K. Crozat, P. Krebs, R. Arens, D. Popkin, C. Arnold, et al., Flt3 permits survival during infection by rendering dendritic cells competent to activate nk cells, Proceedings of the National Academy of Sciences, vol. 107, no. 21, p. 9759-9764, 2010. https://doi.org/10.1073/pnas.1005186107
[10] P. Tsapogas, L. Swee, A. Nusser, N. Nuber, M. Kreuzaler, G. Capoferri, et al., In vivo evidence for an instructive role of fms-like tyrosine kinase-3 (flt3) ligand in hematopoietic development, Haematologica, vol. 99, no. 4, p. 638-646, 2014. https://doi.org/10.3324/haematol.2013.089482
[11] K. Tse, G. Mukherjee, & D. Small, Constitutive activation of flt3 stimulates multiple intracellular signal transducers and results in transformation, Leukemia, vol. 14, no. 10, p. 1766-1776, 2000. https://doi.org/10.1038/sj.leu.2401905