Most eukaryotic cells depends on an internal clock for survival; they proceed through a sequential event known as the cell
cycle . The cell cycle allows multicellular organisms to grow and divide and single-celled organisms to reproduce. However, aberrations in normal cell cycle regulation often result in inappropriate cell
division, eventually causing diseases even carcinogenesis. So the research on cell cycle markers has attracted more and more attention in recent years.
In this post, we will introduce cell cycle markers from five aspects, including the cell cycle, the definition of cell cycle markers, cell cycle markers and their
functions, cell cycle marker-associated products, and applications of cell cycle markers in tumors.
1. What Is the Cell Cycle?
The cell cycle is the whole process that the continuously proliferating cell undergoes from the completion of one division to
the end of the next division. It covers a highly ordered and regulated series of events that results in eukaryotic cell reproduction. Morphologically, it is subdivided into the interphase and mitotic (M) phases.
The interphase consists of the first growth (G1), DNA synthesis (S), and the second growth (G2) phases.
The G1 phase is a regulatory period during which a series of events determine whether cells continue to divide or exit from the cell cycle (entry into
quiescent (G0) phase). In the late G1 phase, cells begin to synthesize the mRNA, protein, and enzymes needed for entry into the S phase. DNA completes replication in the S phase, so the chromosome
contains two sister chromatids attached at the centromere. The G2 phase is the final preparation for cell division. Centrioles have been duplicated, forming two centrosomes. RNA and tubulin are also
synthesized. The M phase includes chromosomal segregation, nuclear division, and cell division. Two genetically identical daughter cells have been created. The rigorous control of the M phase is essential
for the successful completion of sister-chromatid segregation and cell division.
2. What Are Cell Cycle Markers?
During a complete cell cycle, cyclin-dependent kinase (CDK) activation by cyclins phosphorylates related proteins, leading to
the activation and degradation of other proteins, thus allowing for the right transition of one phase from the next one. Those proteins activated or degraded in the specific stage of the cell cycle are denoted
cell cycle markers. They can provide an assessment of cell cycle progression and cell cycle phase distribution.
3. Cell Cycle Marker Family Members and Their Functions
Cell cycle markers are resopnsible for the modulation and maintenance of the cell cycle of eukaryotic cells. They include E2F transcription factors, cyclin-dependent kinases
(CDKs), cyclins, minichromosome
maintenance (MCM) proteins, proliferating cell nuclear antigen (PCNA), cell division cycle 25 (Cdc25) proteins, and Geminin. Cyclin D-Cdk4/Cdk6 regulates events in the early G1 phase; cyclin E-Cdk2
triggers S phase; MCM proteins are essential for initiating DNA replication; cyclin A-Cdk2 and cyclin A-Cdk1 regulate the completion of S phase; all three Cdc25 proteins function during mitosis; Cdk1-cyclin
B is responsible for mitosis.
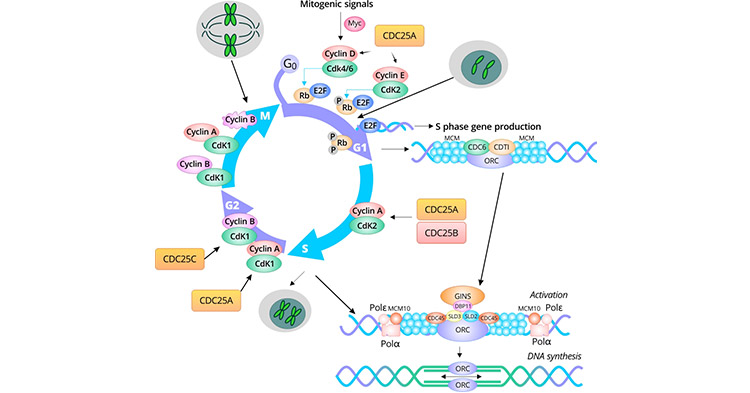
Figure: Cell cycle markers at each phase of the cell cycle
G1-to-S
In mammalian cells, pocket proteins retinoblastoma (RB) protein, p107, and p130 bind to the E2F transcription factors, thus
inhibiting transcription during the early G1 phase. E2F1, E2F2, and E2F3 are found to be combined with RB during G1 [1]. E2F4 and E2F5 are found to be complexed with p130 in the quiescent
(G0) phase and p107 and p130 in the G1 phase. The suppressive roles of E2F6, E2F7, and E2F8 are independent of pocket proteins. E2F6 accumulates when cells shift to the S phase and binds to target
promoters to inhibit transcription.
Mitogens stimulate the Erk-dependent activation of gene regulatory protein Myc, which increases the gene expression, including Cyclin D, SCF subunit
gene, and E2F gene. Cyclin D is required for G1/S cell cycle transition. Cyclin D1 forms a complex with Cdk4 or Cdk6, leading to the phosphorylation of Rb. Up-regulation of the transcription of the SCF
subunit gene promotes the degradation of the CKI protein p27 thus increasing cyclin E-Cdk2 activity [2]. Phosphorylated RB becomes inactive, leading to the liberation of E2F proteins, thus
inducing the expression of genes involved in G1-S transition, including c-Myc, cyclin D1, cyclin A, cyclin E, p21, Cdk2, Cdc2, PNCA, and Cdc25C. RB remains inactive until the M phase it is dephosphorylated
by pp1-type phosphatase. Cyclin E-Cdk2 and cyclin A/Cdk2 phosphorylate RB, reinforcing a positive feedback loop. In the late G1 phase, Cdc25A is highly expressed and activates cyclin D/Cdk4/6 and cyclin
E/Cdk2, accelerating the G1/S transition [3].
Minichromosome maintenance (MCM) is a highly conserved protein family originally found in yeast. MCM proteins are necessary DNA replication
factors essential for initiating DNA replication only once per cell cycle in eukaryotes [2]. In the early G1 phase, DNA replication licensing factor cell division cycle 6 (Cdc6) and chromatin
licensing and DNA replication factor 1 (Cdt1) is recruited to the replication origin by the ORC (ORC1-6) and then load the MCM2–7 hexamer onto chromatin to form the "licensed" (L) pre-replication complex
(pre-RC) [2] [4] [5]. Geminin, a DNA licensing repressor, is the initial signal of the transformation from the G1 phase to the S phase. At the initial stage of the S phase, Geminin begins to
accumulate in the nucleus. With the advance of the cell cycle, Geminin interferes with the stability of pre-RC by binding to Cdt1, and prevents MCM from loading onto the chromosome, thus causing the
termination of DNA replication initiation. It accumulates in the G2 phase and peaks at the M phase, with enhanced complex (APC) activity during anaphase, which induces a "destruction box" mutation of
the Geminin gene and degrades before the next G1 phase. In the late G1 phase, the helicase activity of the MCM complex is activated by the Cdc45/MCM2–7/GINS complex. In the S phase, the activated
MCM complex unwinds the duplex DNA at the origins, recruits DNA polymerases (Polε and Polδ), and initiates DNA synthesis. During the S phase, cyclin A/Cdk2 is active [5].
Proliferating cell nuclear antigen (PCNA) is required for DNA replication and repair. As a cofactor of DNA pol δ/ɛ, it exerts roles in the DNA synthesis
and the maturation of the Okazaki fragments. It is inducibly expressed in late G1, peaks in S-phase, and is reduced thereafter. PCNA has been widely used as a marker of the S phase [6] [7].
There exist three Cdc25 phosphatases in higher-eukaryotic cells: Cdc25A, Cdc25B, and Cdc25C. Cdc25A mainly functions to regulate the G1/S transition [8].
Cdc25B acts as a mitotic initiator and Cdc25C assists in triggering cell entry into mitosis by dephosphorylating cyclin B/Cdk1 [3] [9] [10].
S-to-G2
Cyclin A2 regulates the S/G2 transition and is also involved in mitotic entry [11]. It presents during S, G2, and
early M phases. Cyclin A2-Cdk2 plays a role in initiating the mitotic kinase PLK1 activation during the G2 phase. Later in G2, Cyclin A2-Cdk1 and Cyclin B-Cdk1 can further increase activation of PLK1 through
phosphorylation of Bora, eventually resulting in a commitment to M phase and protection of Bora from SCF-dependent degradation [12] [13].
G2-to-M
The G2-to-M progression is the most profound morphologic and physiological change that takes place during the life of
proliferating cells. The Cyclin B/Cdk1 complex is essential for the transition of the G2 to M phase. It has three subtypes in mammals: B1, B2, and B3. Centrosome-associated checkpoint kinase 1 (CHK1)
phosphorylates Cdc25B, which evokes cyclin B/Cdk1 activity and modulates centrosomal microtubule nucleation at the G2-M transition [9]. Cdc25A plays a more extensive role in assisting both
G1/S and G2/M progression by dephosphorylating Cdk4, Cdk6, as well as Cdk2 and Cdk1.
Cyclin B has three subtypes in mammals: B1, B2, and B3. Cyclin B1 forms an M-phase promoting factor (MPF) complex with Cdk1, and its expression
reaches a peak in the late G2 phase. Cdc25C stimulates cyclin B1/Cdk1 activity, promoting the G2/M transition [9]. Cyclin B1 rapidly degrades and disappears at the middle telophase of
mitosis. At the same time, cells exit the M phase and enter the next cell cycle. Cyclin B2-Cdk1 binds to the Golgi apparatus during interphase and may act in Golgi disassembly during mitosis.
Aurora A/B and PLK1 accumulate during the S phase and reach a peak in the G2/M phase, followed by a rapid degradation at the end of mitosis [14].
Histone H3 is a substrate for the Aurora kinases and is phosphorylated on Serine 10 only in mitosis [15]. As the cell cycle progresses,
H3S10PH reaches a peak in metaphase and extends to all parts of the chromosome. When the cell mitosis enters anaphase and telophase, H3S10PH will appear in the central part of the spindle until the
end of mitosis and the dephosphorylation of H3S10PH occurs. This means that H3S10PH persists throughout the M phase and plays an important role in chromosome aggregation and segregation during the
M phase. Since H3S10PH is significantly associated with the late G2 phase to the M phase of the cell cycle, it has been used as a specific marker for the M phase in some studies targeting the mitotic phase
of tumor cells.
4. Applications of Cell Cycle Markers in Tumors
The cell cycle is a tightly regulated process that inflects the growth, development, and replication of all eukaryotic cells.
Deregulation of the cell cycle is associated with many diseases, especially cancer. Cancerous changes in a normal cell can result from abnormal DNA replication and DNA repair mechanisms. So some cell
cycle markers have important application prospects in the diagnosis and prognosis evaluation of tumors.
Since MCM proteins play important roles in genome duplication in proliferating cells, deregulation of their function results in chromosomal defects that
may contribute to tumorigenesis. The accurate progression of the G1-to-S transition is vital to eukaryotic cell proliferation. Deregulation of the G1 progression is a frequent occurrence in cancer. MCM3 is
indispensable for the initiation of DNA replication and plays an important role in ensuring precise DNA replication initiation once per cell cycle. High expression of MCM5 has been found in diverse human
tumors, such as cervical cancer, skin cancer, and pancreatic cancer.
Cyclins A and E are potential to be markers for diagnosis and prognosis in human cancer. Cyclin A1 is a tissue-specific cyclin highly expressed in acute
myeloid leukemia and testicular cancer. Cyclin A2 is associated with cellular proliferation and can be used for molecular diagnostics as a proliferation marker. Besides, cyclin A2 expression is related to a
poor prognosis in several types of cancer. High levels of cyclin E expression are found in many types of cancer. Cyclin E overexpression is not only associated with proliferation but rather indicates a more
malignant phenotype likely to be linked to the induction of chromosomal instability. These biological functions of cyclin E relate to a poor prognosis when high cyclin E levels are found. The link between
cyclin E and poor prognosis is well established in breast and lung cancer but is likely to be observed in other cancers as well [16].
Cdc25A overexpression has been frequently reported in multiple cancer cell lines, which is highly associated with malignancy and poor prognosis in
cancer patients [8].
5. Cell Cycle Marker-associated Products
Mounting studies have focused on cell cycle markers due to their important roles in the evaluation of tumor progression and prognosis. Demand for products related to cell
cycle markers has also increased. CUSABIO has been committed to providing high-quality scientific research products for the majority of scholars and scientific institutions. Here is an list of cell cycle
marker-associated products.
References
[1] Cosetta Bertoli, Jan M. Skotheim, et al. Control of cell cycle transcription during G1 and S phases [J]. Nat Rev Mol Cell Biol. 2013 Aug; 14(8):
518–528.
[2] Qian Wei, Junhui Li, et al. Phosphorylation of Minichromosome Maintenance Protein 7 (MCM7) by Cyclin/Cyclin-dependent Kinase
Affects Its Function in Cell Cycle Regulation [J]. CELL BIOLOGY VOLUME 288, ISSUE 27, P19715-19725, JULY 05, 2013.
[3] Swastika Sur and Devendra K. Agrawal. Phosphatases and Kinases Regulating CDC25 Activity in the Cell Cycle: Clinical Implications of
CDC25 Overexpression and Potential Treatment Strategies [J]. Mol Cell Biochem. 2016 May; 416(1-2): 33–46.
[4] Zheng Li and Xingzhi Xu. Post-Translational Modifications of the Mini-Chromosome Maintenance Proteins in DNA Replication [J]. Genes
2019, 10(5), 331.
[5] M Loddo, S R Kingsbury, et al. Cell-cycle-phase progression analysis identifies unique phenotypes of major prognostic and predictive
significance in breast cancer [J]. British Journal of Cancer volume 100, pages959–970(2009).
[6] Emily Ming-Chieh Lu, Jithendra Ratnayake, et al. Assessment of proliferating cell nuclear antigen (PCNA) expression at the invading
front of oral squamous cell carcinoma [J]. BMC Oral Health volume 19, Article number: 233 (2019).
[7] Wojciech Strzalka and Alicja Ziemienowicz. Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle
regulation [J]. Ann Bot. 2011 May; 107(7): 1127–1140.
[8] Tao Shen and Shile Huang. The role of Cdc25A in the regulation of cell proliferation and apoptosis [J]. Anticancer Agents Med Chem. 2012
Jul; 12(6): 631–639.
[9] Estelle Schmitt, Rose Boutros, et al. CHK1 phosphorylates CDC25B during the cell cycle in the absence of DNA damage [J]. Journal of
Cell Science 2006 119: 4269-4275.
[10] Kai Liu, Minying Zheng, et al. The role of CDC25C in cell cycle regulation and clinical cancer therapy: a systematic review [J].
Cancer Cell International volume 20, Article number: 213 (2020).
[11] Helena Silva Cascales, Kamila Burdova, et al. Cyclin A2 localises in the cytoplasm at the S/G2 transition to activate PLK1 [J]. Life
Sci Alliance. 2021 Jan 5;4(3):e202000980.
[12] Gheghiani, L., D. Loew, B. Lombard, et al. PLK1 Activation in Late G2 Sets Up Commitment to Mitosis [J]. Cell Rep. 2017, 19:2060–
2073.
[13] Tavernier, N., A. Noatynska, C. Panbianco, et al. Cdk1 phosphorylates SPAT-1/Bora to trigger PLK-1 activation and drive mitotic entry
in C. elegans embryos [J]. J. Cell Biol. 2015, 208:661–669.
[14] Kulkarni AA, Loddo M, et al. DNA replication licensing factors and aurora kinases are linked to aneuploidy and clinical outcome in
epithelial ovarian carcinoma [J]. Clin Cancer Res 2007, 13: 6153–6161.
[15] Crosio C, Fimia GM, et al. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases P [J].
Mol Cell Biol 2002, 22: 874–885.
[16] Amber Yasmeen, Wolfgang E Berdel, et al. E- and A-type cyclins as markers for cancer diagnosis and prognosis [J]. Expert Rev Mol
Diagn. 2003 Sep;3(5):617-33.
CUSABIO team. A Group of Promising Proteins - Cell Cycle Markers. https://www.cusabio.com/c-21021.html




Comments
Leave a Comment