Recently, there has been a flurry of good news regarding TNFSF15/TL1A. Sansheng Guojian Pharmaceutical(Group)Co., Ltd.'s anti-TL1A monoclonal antibody SSGJ-627 injection has had its IND application accepted by the CDE, marking the beginning of clinical research on the TL1A target by Chinese pharmaceutical companies. At the 43rd J.P. Morgan Annual Healthcare Conference, Merck estimated the potential of the TL1A target in autoimmune treatment to be worth billions of dollars. Teva Pharmaceutical also expressed satisfaction with the clinical data of its anti-TL1A monoclonal antibody, duvakitug.
In the trading market, the TL1A target has also been a focal point. In 2023, Merck acquired Prometheus for $10.8 billion. The core product of Prometheus is a TL1A - targeting monoclonal antibody. Sanofi and Teva reached a $1 billion collaboration to develop Teva's TL1A antibody therapy, tev'574. In December, Roche acquired Telavant for $7.1 billion in advance payments, gaining the rights to rvt - 3101 in the US and Japan. In June 2024, MingJi Bio reached a collaboration with AbbVie worth approximately $1.71 billion to develop the next - generation TL1A antibody, fg - m701, for the treatment of inflammatory bowel disease.
The reason behind the rush of pharmaceutical companies to layout the TL1A target is worth exploring in depth. As an important member of the tumor necrosis factor superfamily, TL1A plays a central role in key physiological and pathological processes such as immune regulation, inflammatory response, and the development of tumors. Below, let's delve into the application and progress of TL1A in drug development from aspects such as its biological characteristics, signaling pathways, and associations with related diseases.
1. What is TNFSF15/TL1A
1.1 Gene and Protein Structure
TNFSF15, also known as TL1A, is a type II transmembrane protein composed of an intracellular domain, a transmembrane domain, and an extracellular domain. The extracellular domain contains a conserved TNF homology domain, which is the key part for TNFSF15 to bind with receptors and directly determines its biological activity [1]. Moreover, the extracellular domain can be enzymatically cleaved into a soluble form, which functions differently from the membrane - bound form in the body.
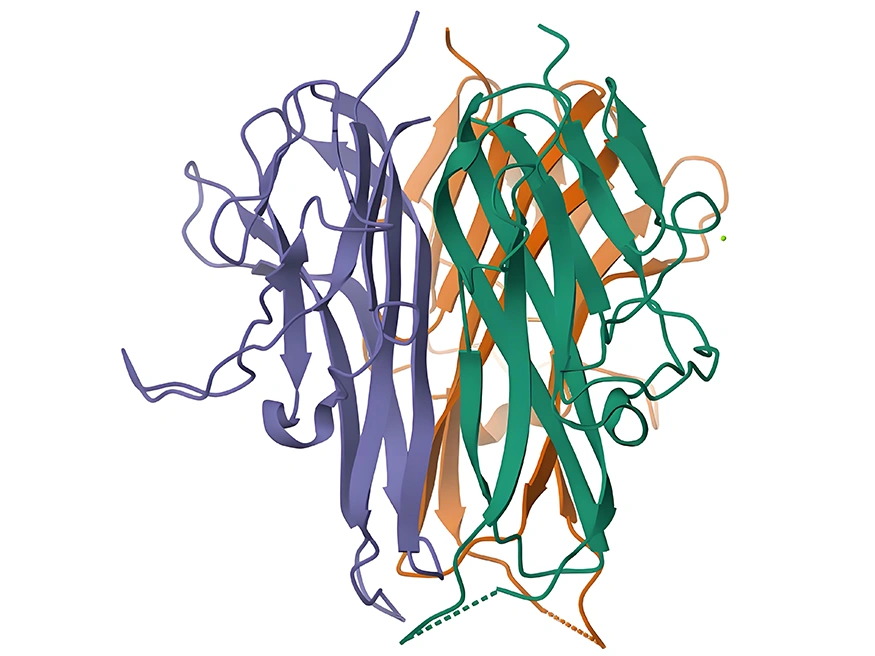
Figure 1: Schematic diagram of TNFSF15 protein structure
(Source: www.rcsb.org)
The TNFSF15 gene consists of 5 exons and 4 introns. In its encoded protein, the intracellular domain may be involved in the initiation and regulation of intracellular signaling, the transmembrane domain anchors the protein to the cell membrane, and the TNF homology domain in the extracellular domain plays a decisive role in receptor binding and activation of downstream signaling [1,2]. The TNFSF15 gene and protein sequences show a certain degree of conservation across different species, implying the importance of its function and evolutionary conservation.
1.2 Expression and Regulatory Mechanisms
TNFSF15 is expressed in various immune cells, such as T cells, B cells, and monocytes. Its expression is precisely regulated by multiple factors, including inflammatory cytokines and transcription factors. Under inflammatory conditions, cytokines like TNF - α and IL - 1β can bind to their respective receptors on the cell surface, activating intracellular signaling pathways that upregulate TNFSF15 expression, thus participating in the cascade amplification of inflammatory responses [2,3]. Additionally, transcription factors such as NF - κB can directly bind to the promoter region of the TNFSF15 gene to regulate its transcription level.
1.3 Related Signaling Pathways
TNFSF15 mainly activates signaling pathways by binding to its receptor DR3. After their binding, the adaptor protein TRADD is recruited, which then activates multiple signaling pathways, including NF - κB and MAPK. The activation of the NF - κB signaling pathway promotes the expression of a series of inflammatory cytokines and anti - apoptotic proteins, affecting cell survival, proliferation, and inflammatory responses. The MAPK signaling pathway, conversely, plays an important regulatory role in cell proliferation, differentiation, and apoptosis [2,3].
2. Association of TNFSF15/TL1A with Diseases
2.1 TNFSF15/TL1A and Autoimmune Diseases
● Inflammatory Bowel Disease (IBD)
Inflammatory bowel disease (IBD), primarily encompassing Crohn's disease and ulcerative colitis, is a group of chronic non - specific intestinal inflammatory disorders with unclear etiologies. Abundant studies have demonstrated that TNFSF15 gene polymorphisms are closely linked to the susceptibility to IBD [7]. In the intestinal tissues of IBD patients, the expression of TNFSF15 is remarkably upregulated. Mechanistically, TNFSF15 plays a pivotal role in the intestinal inflammatory cascade and fibrosis via its DR3 receptor. It activates the NF - κB signaling pathway, resulting in the massive release of inflammatory cytokines like IL - 6, IL - 8, and TNF - α, thereby triggering intestinal mucosal inflammation and immune dysregulation [3,7]. Clinical investigations have revealed that the levels of TNFSF15 in the serum and intestinal mucosa of IBD patients are positively correlated with disease activity, making it a potential biomarker for evaluating disease severity and prognosis.
Multiple genetic studies on IBD patients of diverse ethnicities have identified specific single - nucleotide polymorphisms (SNPs) in the TNFSF15 gene that are associated with an increased disease risk [7]. In animal experiments, knocking out the TNFSF15 gene or blocking its signaling pathway significantly alleviated the symptoms of experimental colitis in mice. This was manifested as reduced intestinal mucosal damage and decreased expression of inflammatory cytokines. Furthermore, the application of neutralizing TNFSF15 antibodies effectively reversed colonic fibrosis, restoring it to the pre - inflammatory state. This reversal might be ascribed to the decreased expression of connective tissue growth factor, IL31Ra, TGF - β1, and IGF - 1.
● Rheumatoid Arthritis (RA)
Rheumatoid arthritis is an autoimmune disease mainly characterized by synovial inflammation of the joints. It can lead to joint pain, swelling, and deformity, severely impairing patients' quality of life. In patients with rheumatoid arthritis, the levels of TNFSF15 are significantly elevated [4]. It is capable of stimulating the proliferation of synovial cells and promoting the secretion of inflammatory cytokines such as IL - 1, IL - 6, and TNF - α. These cytokines further activate immune cells, forming an inflammatory vicious cycle that causes cartilage and bone destruction in the joints [4]. Research has also discovered that TNFSF15 can induce the differentiation and activation of osteoclasts, accelerating joint bone resorption and exacerbating joint damage. Clinical studies have indicated that the serum TNFSF15 levels in rheumatoid arthritis patients are correlated with disease activity indices and the extent of joint destruction [4].
2.2 TNFSF15/TL1A and Tumors
● Impact on Tumor Angiogenesis
The growth and metastasis of tumors depend on an adequate blood supply, and angiogenesis plays a crucial role in this process. TNFSF15 can facilitate tumor angiogenesis by regulating the expression of angiogenic factors, such as vascular endothelial growth factor (VEGF) [5,8]. Studies have found that TNFSF15 can activate relevant signaling pathways in tumor cells or within the tumor microenvironment, upregulating the expression and secretion of VEGF [5,8]. When VEGF binds to its receptors, it promotes the proliferation, migration, and lumen formation of vascular endothelial cells, providing nutritional support for tumor growth and metastasis. In various solid tumors, including breast cancer, lung cancer, and colorectal cancer, a close relationship between TNFSF15 and tumor angiogenesis has been observed.
Clinical studies have shown that the expression levels of TNFSF15 in tumor tissues are positively correlated with tumor microvascular density. Tumor patients with high TNFSF15 expression usually have a poorer prognosis [8]. In animal experiments, inhibiting the function of TNFSF15 significantly reduced tumor angiogenesis and inhibited tumor growth and metastasis.
● Tumor Immune Microenvironment
The tumor immune microenvironment represents a complex ecosystem that consists of tumor cells, immune cells, stromal cells, and the extracellular matrix. TNFSF15 exerts a dual - regulatory function within the tumor immune microenvironment [6].
On one hand, TNFSF15 is capable of activating T cells and natural killer (NK) cells, thereby enhancing the body's anti - tumor immune response [6]. It promotes the proliferation, differentiation, and cytokine secretion of T cells, which in turn augments their cytotoxic activity against tumor cells. Simultaneously, it also activates NK cells, enabling them to exert more potent cytotoxic effects.
On the other hand, in specific tumor microenvironments, TNFSF15 can trigger the generation and accumulation of immunosuppressive cells, such as regulatory T cells (Tregs) and myeloid - derived suppressor cells (MDSCs), facilitating tumor immune evasion.
Clinical studies have revealed that in some tumor patients, the expression levels of TNFSF15 in tumor tissues are positively correlated with Treg cell infiltration and are associated with an unfavorable prognosis [6]. Conversely, in other tumors, high TNFSF15 expression is linked to better immune infiltration and prognosis [6]. This indicates that the role of TNFSF15 in the tumor immune microenvironment may differ depending on factors such as tumor type, stage, and individual variations.
3. Research and Development Progress of TNFSF15/TL1A-Targeted Drugs
Currently, several monoclonal antibodies targeting TNFSF15 have entered clinical trial stages. These antibody drugs mainly work by specifically binding to TNFSF15, blocking its interaction with the receptor DR3, thereby inhibiting downstream signaling to achieve therapeutic effects. The main indications are ulcerative colitis, Crohn's disease, etc., with the highest research stage being Phase 3. Some are listed in the table below:
| Drug Name |
Drug Type |
Indications for Research |
Research Institutions |
Highest Research Stage |
| Afimkibart |
Monoclonal Antibody |
Active Moderate Ulcerative Colitis, Active Severe Ulcerative Colitis, Ulcerative Colitis, Crohn's Disease |
Hoffmann-La Roche, Inc. | F. Hoffmann-La Roche Ltd. | Hoffmann-La Roche Ltd. | Roivant Sciences Ltd. | Roche Holding AG |
Phase 3 |
| Tulisokibart |
Monoclonal Antibody |
Crohn's Disease, Ulcerative Colitis, Diffuse Scleroderma, Systemic
Sclerosis-Associated Interstitial Lung Disease, Active Moderate Ulcerative Colitis, Active Severe Ulcerative Colitis |
Prometheus Biosciences, Inc. | Merck Sharp & Dohme Corp. | Merck Sharp & Dohme LLC |
Phase 3 |
| Duvakitug |
Monoclonal Antibody |
Ulcerative Colitis, Crohn's Disease |
Sanofi | Teva Branded Pharmaceutical Products R&D, Inc. |
Phase 2 |
| XmAb942(Xencor) |
Monoclonal Antibody |
Ulcerative Colitis |
Xencor, Inc. |
Phase 1/2 |
| BCD-261 |
Monoclonal Antibody |
- |
Biocad CJSC |
Phase 1 |
| PF-07261271 |
Bispecific Antibody |
Inflammatory Bowel Disease |
Pfizer Inc. |
Phase 1 |
| SPY-002 |
Monoclonal Antibody |
Inflammatory Bowel Disease |
Spyre Therapeutics, Inc. |
Phase 1 |
| SSGJ-627 |
Monoclonal Antibody |
- |
SanSheng GuoJian Pharmaceuticals (Shanghai) Co., Ltd. |
IND Application |
In the field of autoimmune diseases, preliminary clinical trial results show that these TNFSF15-targeting antibody drugs have good efficacy and safety. For example, in clinical trials for inflammatory bowel disease, treatment with these antibody drugs significantly improved the intestinal inflammation symptoms of patients, reduced disease activity, and caused only mild adverse reactions.
In the field of tumor treatment, although TNFSF15-targeting antibody drugs are still in the exploratory stage, studies have shown that their combination with other anti-tumor treatments such as chemotherapy and immunotherapy can enhance anti-tumor effects [1,2].
4. Product Recommendations for TNFSF15/TL1A Research
To support the research on TNFSF15/TL1A by pharmaceutical companies and research institutions, CUSABIO has launched a range of high-activity TNFSF15/TL1A protein products, covering multiple species including human, mouse, and monkey. Additionally, we provide TNFSF15/TL1A antibodies and ELISA kits to assist your research on the mechanisms of TNFSF15/TL1A or the exploration of its potential clinical value.
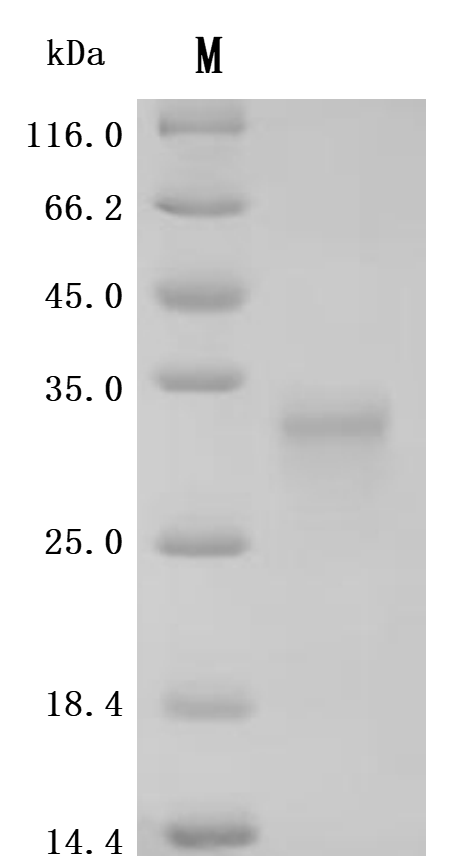
● TNFSF15/TL1A Recombinant Proteins
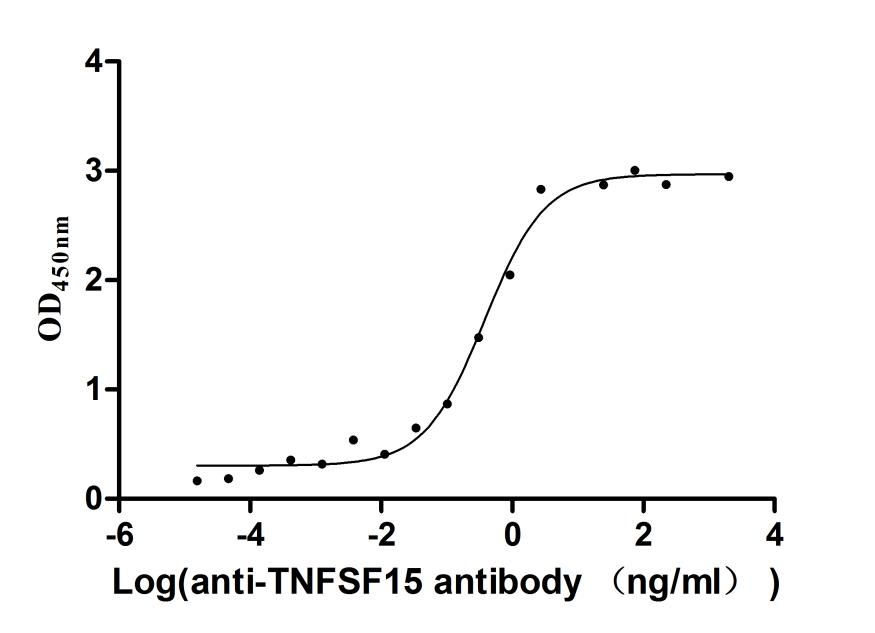
● TNFSF15/TL1A Antibodies
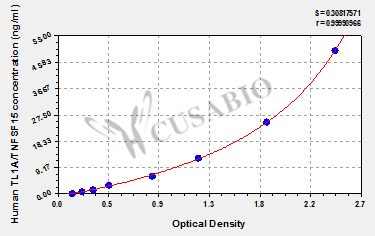
● TNFSF15/TL1A ELISA Kits
For more information, please visit our website to view all TNFSF15/TL1A-related products.
References
[1] Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76(6):959 - 962.
[2] Aggarwal BB. Signaling pathways of the TNF - superfamily: a double - edged sword. Nat Rev Immunol. 2003;3(9):745 - 756.
[3] Neurath MF. TNFalpha in inflammatory bowel disease: friend or foe? Trends Mol Med. 2003;9(11):518 - 524.
[4] Chabaud M, Fossiez F, Taupin JL, et al. Overexpression of interleukin - 15 in rheumatoid synovium and in interleukin - 1 - stimulated rheumatoid synovial fibroblasts. Arthritis Rheum. 1996;39(4):681 - 689.
[5] Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669 - 676.
[6] Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991 - 998.
[7] Zhang X, Li Y, Wang Y, et al. TNFSF15 polymorphisms and susceptibility to inflammatory bowel disease: a meta - analysis. Inflamm Bowel Dis. 2012;18(10):1864 - 1873.
[8] Yang Z, Wang Y, Li Y, et al. TNFSF15 promotes angiogenesis in colorectal cancer by upregulating VEGF expression. Oncol Rep. 2015;33(2):671 - 673.
CUSABIO team. TNFSF15/TL1A: The Rise of the Next Billion-Dollar Target as Pharma Giants Stake Their Claims. https://www.cusabio.com/c-21208.html

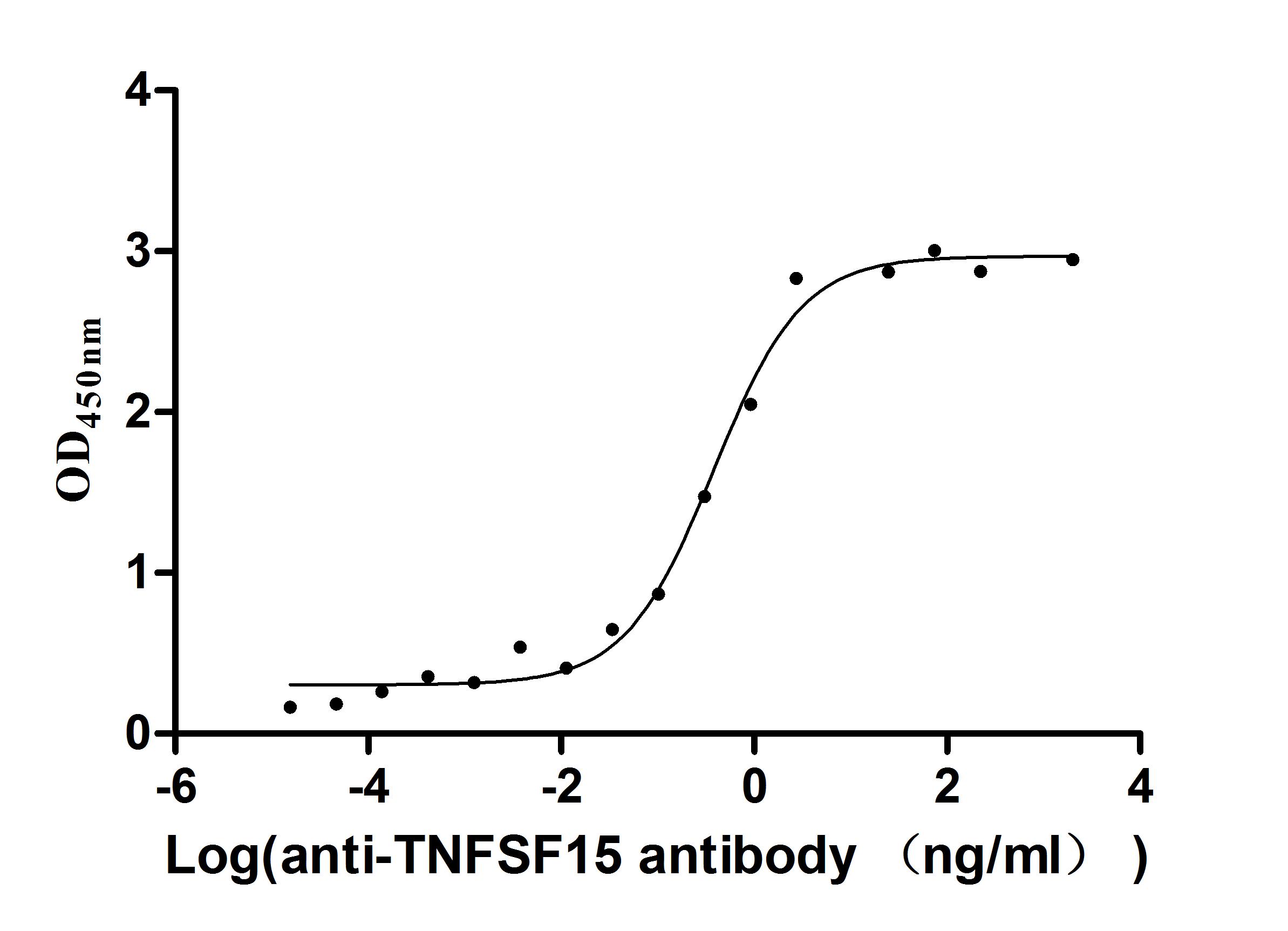

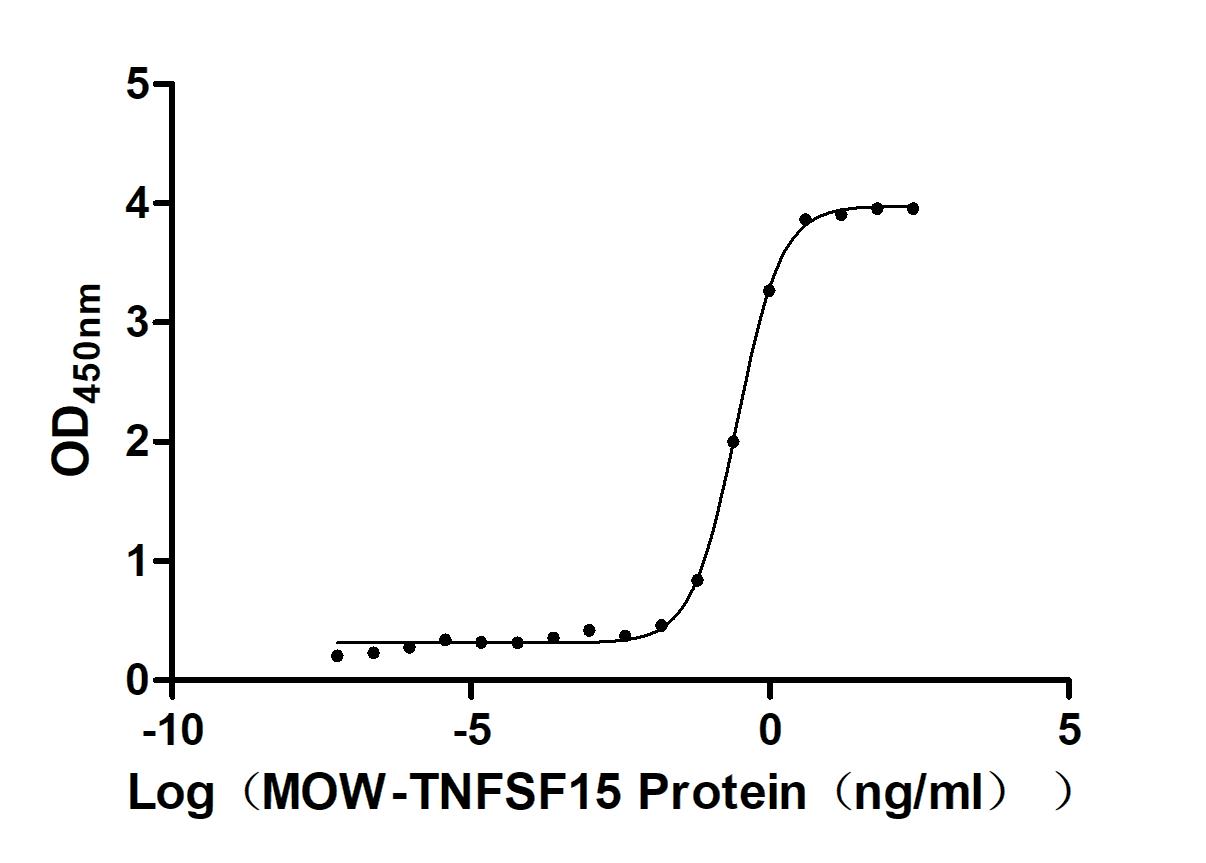
-AC1.jpg)
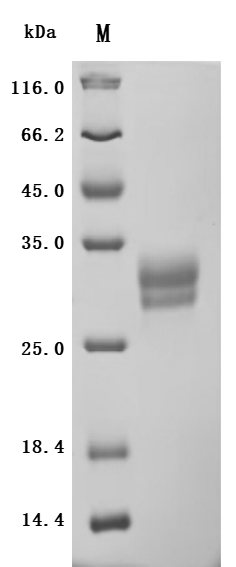
-SDS.jpg)




Comments
Leave a Comment