1. Background and Basic Functions of PVR
PVR (CD155), a member of the immunoglobulin superfamily, is a cell surface glycoprotein initially identified as the receptor for poliovirus, hence its name Poliovirus Receptor (PVR). It is widely distributed in various tissues and cell types, including epithelial cells, nerve cells, and immune cells. Under normal physiological conditions, PVR performs functions such as cell adhesion and signal transduction, playing a vital role in maintaining normal physiological functions and the integrity of tissue structure.
In the immune system, PVR interacts with multiple receptors to regulate immune cell functions, including the activating receptor DNAM-1 (CD226) and the inhibitory receptors TIGIT and CD96, forming a complex immune regulatory network. PVR is lowly expressed in normal tissues but significantly upregulated in various tumors, such as acute myeloid leukemia, multiple myeloma, and breast cancer, making it a potential target for immunotherapy.
2. Molecular Mechanisms and Signaling Pathways of PVR
2.1 Immune Regulatory Network
● TIGIT/PVR Axis
TIGIT, a high-affinity receptor of PVR, is primarily expressed on NK cells, T cells, and regulatory T cells (Tregs). Upon binding to TIGIT, PVR recruits and activates SHP-1/SHP-2 phosphatases, thereby inhibiting NK cell cytotoxicity and T cell activation, inducing immune exhaustion, and promoting tumor immune evasion. For instance, in acute myeloid leukemia (AML), PVR overexpression is associated with the exhaustion of TIGIT+ CD8+ T cells, and blocking the TIGIT/PVR axis can restore T cell proliferation and cytokine secretion [1-3].
● DNAM-1/PVR Axis
DNAM-1, an activating receptor of PVR, mediates the activation of NK cells and T cells. Tumor cells can suppress immune cell function by downregulating DNAM-1 or upregulating PVR. For example, in multiple myeloma (MM), PVR overexpression is linked to weakened NK cell cytotoxicity mediated by DNAM-1.
● CD96/PVR Axis
CD96 binding to PVR inhibits NK cell activity and acts synergistically with TIGIT to contribute to the immunosuppressive tumor microenvironment.
2.2 Signaling Pathways
● NF-κB and MAPK Pathways
PVR promotes tumor cell proliferation and migration by activating the Ras-Raf-MEK-ERK signaling pathway. In glioblastoma, PVR overexpression enhances tumor invasiveness by upregulating MMP-9 via the NF-κB pathway.
● Epigenetic Regulation
The methylation status of the PVR promoter affects its expression. In liver cancer, PVR promoter hypomethylation leads to its overexpression, which is associated with poor prognosis. DNA methylation inhibitors, such as 5-aza-2’-deoxycytidine, can restore PVR expression and enhance the sensitivity of TIGIT blockade therapy [4].
3. PVR and Associated Diseases
3.1 Tumor Diseases
PVR is upregulated on the surface of various tumor cells. Its high expression, combined with TIGIT binding, creates an immunosuppressive microenvironment, suppressing anti-tumor immune responses and correlating with tumor progression, metastasis, and poor patient prognosis in cancers like non-small cell lung cancer and colorectal cancer. In acute myeloid leukemia (AML), PVR overexpression on CD8+ T cells is associated with T cell exhaustion. PVR-4-1BBL fusion proteins can enhance T cell activation and cytokine secretion [5], exhibiting strong anti-tumor activity. In multiple myeloma (MM), PVR overexpression is linked to adverse prognostic factors [6], and blocking the KIR2DL5/PVR pathway along with anti-TIGIT antibodies can enhance NK cell-mediated tumor killing [7-8]. In solid tumors (e.g., breast cancer [9], liver cancer, and colorectal cancer), PVR overexpression is associated with tumor invasiveness, metastasis, and reduced immune cell infiltration. In triple-negative breast cancer, PVR is upregulated via the H19 lncRNA-STAT3 axis, and PVR knockdown can inhibit tumor cell migration and colony formation.
3.2 Neurological Diseases
PVR also plays a significant role in neurological diseases. In multiple sclerosis, PVR regulates immune cell attacks on neural myelin sheaths, influencing inflammatory responses and neurodegeneration. Abnormal PVR expression during nervous system development may be associated with defects in neural cell migration, differentiation, and synapse formation, thereby affecting normal nervous system function.
3.3 Infectious Diseases
Beyond poliovirus infection, PVR is involved in other viral infections, such as HIV. It may serve as a co-receptor for viral entry into immune cells, promoting viral replication and dissemination.
4. Drug Development of PVR
Targeting PVR and its ligands has become a research hotspot in tumor immunotherapy. Several anti-PVR and anti-TIGIT monoclonal antibodies have entered clinical trials and have shown promising anti-tumor effects. For example, anti-TIGIT antibodies can block the PVR-TIGIT interaction, alleviating immune cell inhibition and enhancing anti-tumor immune responses. Lerapolturev (PVSRIPO), an oncolytic virus targeting PVR, is in Phase 2 clinical trials to evaluate its safety and efficacy in advanced solid tumor patients. Below is a list of some ongoing drug development projects:
| Drug |
Drug Type |
Indication |
Developer |
Development Stage |
| Lerapolturev |
Oncolytic Virus |
Glioblastoma Multiforme, Refractory Melanoma, Recurrent Glioblastoma |
Istari Oncology, Inc. | Duke University |
Phase 2 |
| NTX-1088 |
Monoclonal Antibody |
Advanced Malignant Solid Tumors, Tumor Metastasis |
Nectin Therapeutics Ltd. |
Phase 1 |
| Anti-PVR CAR-T cells (Tasrif Pharmaceutical) |
CAR-T |
Tumors |
Tasrif Pharmaceutical |
Preclinical |
| DSP-502 |
Fusion Protein |
Tumors |
KAHR Medical Ltd. |
Preclinical |
| Anti-TAGE monoclonal antibody (Bloom Technology) |
Monoclonal Antibody |
Diabetic Kidney Disease, Diabetic Retinopathy, Non-Alcoholic Fatty Liver Disease |
Bloom Technology Corp. |
Preclinical |
| JS-K (JSK Therapeutics) |
Small Molecule |
Tumors |
JSK Therapeutics, Inc. |
Preclinical |
| NTX-8090 |
ADC |
Solid Tumors |
Nectin Therapeutics Ltd. |
Preclinical |
| TSRF-786C |
Monoclonal Antibody |
Brain Cancer |
Tasrif Pharmaceutical |
Preclinical |
5. Related Product Recommendations
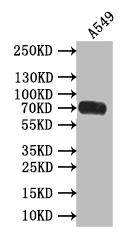
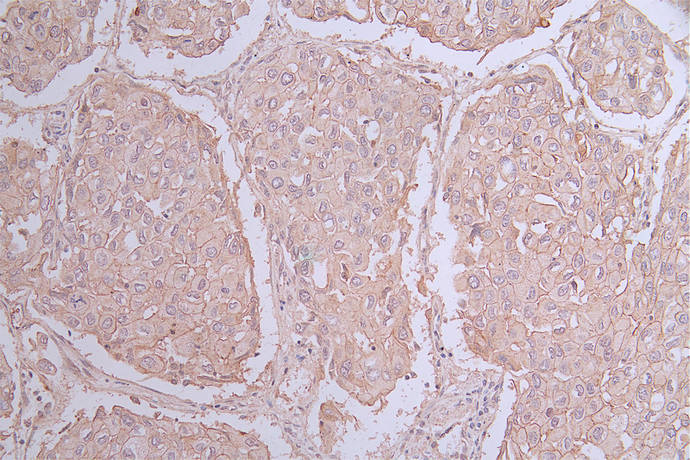
PVR (CD155) plays a crucial role in immune regulation, viral infections, and various diseases. In-depth research on its mechanisms provides new targets for disease diagnosis, treatment, and drug development. Huamei Bio is pleased to introduce high-activity PVR protein products and offers PVR antibodies and ELISA kits to support researchers in studying PVR mechanisms and exploring its potential clinical value.
References
[1] Diong, S. J. J., et al. (2023). Biophysical characterization of PVR family interactions and therapeutic antibody recognition to TIGIT. MAbs, 15(1), 2253788.
[2] Zhou, X., et al. (2021). Computer-aided design of PVR mutants with enhanced binding affinity to TIGIT. *Cell Communication and Signaling, 19(1), 12.
[3] Stengel, K. F., et al. (2012). Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell–cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proceedings of the National Academy of Sciences, 109(14), 5399-5404.
[4] Priglinger, S. G., et al. (2021). Proliferative Vitreoretinopathie (PVR)-Chirurgie: Scar Wars. Der Ophthalmologe, 118(1), 18-23.
[5] Park, H.-Y., et al. (2018). A dual-targeting fusion protein, human PVR-4-1BBL for immunotherapy in AML. Annals of Oncology, 29(suppl_10), x32-x33.
[6] Hattenbach, L.-O., et al. (2021). Proliferative Vitreoretinopathie (PVR) minimal: „Same same, but different“. Der Ophthalmologe, 118(1), 24-29.
[7] Husain, B., & Martinez-Martin, N. (2019).A platform for extracellular interactome discovery identifies novel functional binding partners for the immune receptors B7-H3/CD276 and PVR/CD155. Annals of Oncology, 30(suppl_11), xi37.
[8] Ren, X., et al. (2022). Blockade of the immunosuppressive KIR2DL5/PVR pathway elicits potent human NK cell–mediated antitumor immunity. The Journal of Clinical Investigation, 132(22), e163620.
[9] Elkhouly, A., Soliman, R. A., & Youness, R. A. (2021). Convoluted role of H19 long non-coding RNA in regulating ICAM-1 and PVR in breast cancer patients. Annals of Oncology, 32(s1).
CUSABIO team. PVR (CD155): A Key Factor in Tumor Immune Evasion and a Novel Therapeutic Target. https://www.cusabio.com/c-21221.html

-SDS.jpg)
-AC1.jpg)


Comments
Leave a Comment