B7-H6, also known as Natural Cytotoxicity Triggering Receptor 3 Ligand 1 (NCR3LG1), is a unique member of the B7 co-stimulatory/inhibitory molecule family. It is not constitutively expressed in normal tissues but is significantly overexpressed on the surface of various tumor cells and can be induced in immune cells and damaged tissue cells by inflammatory or infectious signals. As the primary functional ligand for the natural killer (NK) cell activating receptor NCR3 (NKp30), B7-H6 serves as a core molecular switch mediating NK cell recognition of “non-self” and “danger” signals and initiating cytotoxic killing. Its central role in tumor immune evasion, along with its potential functions in infection immunity and inflammatory regulation, makes it a frontier target for tumor immunotherapy and immunomodulatory drug development.
1. Structure, Expression Regulation, and Core Functional Mechanisms of B7-H6
1.1 Molecular Structure and Receptor Binding Specificity
B7-H6 possesses typical B7-family extracellular domains: an N-terminal immunoglobulin variable-like domain (IgV) and a membrane-proximal immunoglobulin constant-like domain (IgC). The IgV domain is the key region for binding NKp30, determining the specificity and affinity of their interaction. Its transmembrane domain anchors it to the cell membrane, while its short intracellular domain suggests its signaling primarily depends on downstream NKp30 pathways. The binding between B7-H6 and NKp30 is highly specific and serves as a critical trigger for NK cell activation.
1.2 Dynamic Expression Regulation
B7-H6 expression is tightly regulated and rarely expressed in normal tissues, but can be potently induced by multiple factors under specific pathological conditions:
● Constitutive high expression in various tumors: Widely present on the surface of solid tumors (e.g., colorectal cancer, gastric cancer, ovarian cancer, breast cancer, melanoma, neuroblastoma, lymphoma, glioma) and hematological malignancies (e.g., leukemia, lymphoma), serving as an important tumor-associated antigen.
● Inducible expression:
(1) Therapy and stress induction: Therapeutic/stress factors such as chemotherapy, radiotherapy, heat shock, and cytokines (TNF-α) significantly upregulate B7-H6.
(2) Integrated Stress Response (ISR): A key induction pathway, driven by PERK-mediated eIF2α phosphorylation. Utilized by specific drugs (HIV protease inhibitors, thapsigargin Tg) and viral infections (e.g., HCMV).
(3) Epigenetic regulation: HDAC3 upregulation promotes expression (can be blocked by HDAC inhibitors); BRD4 binds to the promoter region and cooperates with JMJD6 to recruit the transcriptional machinery, enhancing transcription (can be blocked by the BET inhibitor JQ1).
(4) Transcription factor-driven: The oncogene c-Myc directly upregulates B7-H6 expression, and the two show positive correlation in multiple tumor types.
(5) Inflammatory signals: TLR ligands and inflammatory cytokines (TNF-α, IL-1β) can induce expression in specific immune cells (e.g., inflammatory monocytes, neutrophils).
● Soluble form (sB7-H6): Generated via protease cleavage or alternative splicing, present in serum and body fluids. sB7-H6 is an important immunosuppressive molecule that competitively binds NKp30, blocking membrane-bound B7-H6-mediated NK cell activation. Its serum level correlates with tumor burden and poor prognosis.
1.3 Dual Core Functions: Intrinsic Tumor-Promoting Effects & NK Cell Activation Regulation
1.3.1 Intrinsic Tumor-Promoting Signaling Pathways
B7-H6 is not merely a ligand; its expression itself activates multiple key pro-survival, proliferative, and metastatic signaling pathways within tumor cells:
Ras/MEK/ERK pathway: B7-H6 induces phosphorylation of MEK, ERK, and HIF-1α. Activated ERK1/2 translocates into the nucleus, promoting cancer cell survival, proliferation, migration, and differentiation. B7-H6 downregulation inhibits this pathway.
PI3K/AKT pathway: B7-H6 activates PI3K/AKT signaling. Its downregulation inhibits this pathway, affecting cell survival and metabolism.
STAT3 pathway: B7-H6 induces STAT3 activation, promoting tumorigenesis. Its downregulation reduces levels of STAT3 downstream effectors such as RNMT and c-Myc.
1.3.2 Key Ligand for NK Cell Activation
Activates NK cell killing: Membrane-bound B7-H6 on tumor cell surfaces binds to the activating receptor NKp30 on NK cells, serving as one of the key "activation signals" triggering NK cell cytotoxicity.
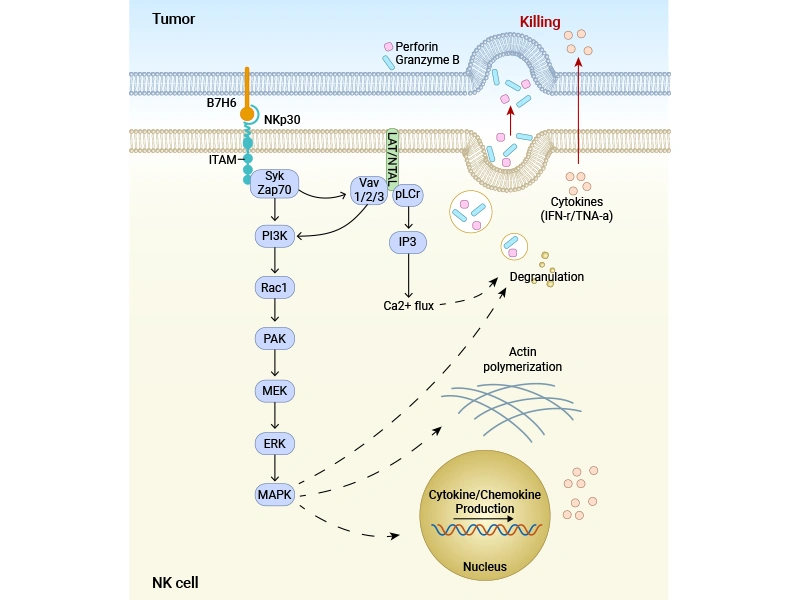
● Signal transduction: Binding leads to phosphorylation of the ITAM (immunoreceptor tyrosine-based activation motif) within the cytoplasmic tail of NKp30 by Src family kinases, which then recruits and activates Syk/ZAP70 kinases.
● Downstream pathway activation:
PLCγ cleaves PIP2 to generate IP3 and DAG, causing increased intracellular Ca²⁺ concentration and PKC activation.
PI3K generates PIP3, activating effector molecules like Akt.
Activates Vav, which in turn activates the small GTPase Rac, leading to MAPK pathway activation via the PAK1-MEK-ERK cascade.
Figure: Schematic diagram of cell lysis induced by B7-H6 binding to NKp30 [1]
2. B7-H6 and Diseases
2.1 B7-H6 and Inflammation
B7-H6 can be induced under inflammatory conditions in non-tumor cells (such as CD14⁺CD16⁺ pro-inflammatory monocytes and neutrophils) and is secreted in a soluble form (sB7-H6). In sepsis, membrane-bound B7-H6 (mB7-H6) activates NK cells by binding to NKp30, exacerbating the early systemic inflammatory response syndrome (SIRS), leading to high mortality. Conversely, exosome-derived sB7-H6 blocks the NKp30 signaling pathway, suppresses NK cell function, promotes immune paralysis in the compensatory anti-inflammatory response syndrome (CARS) phase, and also increases patient mortality risk. Gram-negative bacterial infection specifically induces sB7-H6 production, a mechanism potentially associated with the TLR4-TRIF pathway. The bidirectional immunomodulatory role of B7-H6 (pro-inflammatory/anti-inflammatory) collectively exacerbates the pathological progression of sepsis, suggesting that its dynamic monitoring could serve as a novel indicator for sepsis prognosis stratification [2].
2.2 B7-H6 and Small Cell Lung Cancer (SCLC)
B7-H6 exhibits a unique bidirectional immunomodulatory role in SCLC [3]: Its high expression positively correlates with prolonged progression-free survival (PFS) and increased immune infiltration, but negatively correlates with NK cell activation. Mechanistically, the membrane-bound form of B7-H6 can activate NK cell anti-tumor activity by binding NKp30, while the soluble form inhibits NK function. Compared to PD-L1, B7-H6 shows higher expression in SCLC and greater tumor specificity (with near absence in normal tissues), making it a highly promising novel target for immunotherapy (e.g., ICI, CAR-T). However, its dynamic expression patterns, responsiveness to chemotherapy, and balance with the soluble form require further investigation.
2.3 B7-H6 and T-Lymphoblastic Lymphoma (T-LBL)
B7-H6 was first discovered in T-LBL to undergo nuclear translocation (dependent on the nuclear localization signal NLS and transport proteins XPO-5/6). Its expression significantly correlates with poor prognostic factors such as elevated lactate dehydrogenase, ECOG performance status, and B symptoms, but due to the impact of intensive chemotherapy regimens, it did not directly correlate with patient survival rate [4]. Functionally, B7-H6 drives the disease through dual mechanisms: 1) Membrane/cytoplasmic B7-H6 (accounting for 38.5%) serves as an immunotherapeutic target (NKp30-CAR-T effectively clears tumors in vitro); 2) Nuclear B7-H6 may promote tumorigenesis via non-immune pathways (e.g., regulating the RAG-1 gene), and its expression can be inhibited by histone deacetylase inhibitors. Studies indicate B7-H6 is a potential prognostic marker and therapeutic target in T-LBL, but its tumor-promoting mechanisms require further elucidation.
2.4 B7-H6 and B-Cell Non-Hodgkin Lymphoma (B-NHL)
B7-H6 is highly expressed in B-cell lymphomas and drives malignant progression: Its knockout significantly suppresses tumor proliferation (downregulating Survivin/PCNA/c-Myc) by inhibiting the STAT3 pathway (reducing STAT3/ERK1/2 phosphorylation), arrests the cell cycle at G0/G1 phase (modulating the Cyclin D1-CDK4/6-Rb-p21 axis), promotes apoptosis (decreasing Bcl-2/Bcl-xL, increasing Bax/Bad, activating Caspase-8/3), and enhances chemosensitivity; it also inhibits metastasis (downregulating MMP-2/9). Research demonstrates that B7-H6 cooperatively regulates core malignant phenotypes of lymphoma through the STAT3 signaling axis, positioning it as a potential therapeutic target [5].
2.5 B7-H6 and Hepatocellular Carcinoma (HCC)
B7-H6 expression in HCC positively correlates with tumor volume, while low mRNA levels predict better survival [6]. Functionally, B7-H6 knockout significantly inhibits HCC cell proliferation, migration, and invasion, arrests the cell cycle in G1 phase, and downregulates oncogenes c-Myc/c-Fos and cyclin D1. Mechanistically, B7-H6 exacerbates liver injury (e.g., via IL-32 upregulation) in HBV-associated inflammatory microenvironments through interaction with NKp30, while also directly regulating tumor biological behavior. This suggests B7-H6 plays a dual role as both an inflammation mediator and an oncogene, making it a potential prognostic marker and immunotherapeutic target (e.g., B7-H6-CAR-T) in HCC.
3. Current Status of B7-H6-targeted Drug Development
Database analysis indicates that B7-H6-targeted drugs are still in the early stages of clinical validation and indication exploration/expansion. The most advanced candidate is BI 765049 (developed by Boehringer Ingelheim GmbH), currently in Phase I clinical trials. BI 765049 is a novel immunoglobulin G (IgG)-like T-cell engager (TcE) (bispecific antibody). Its mechanism of action involves simultaneously binding CD3 on T cells and B7-H6 expressed on tumor cells, activating T cells and inducing their killing of tumor cells. It is currently being clinically explored primarily in gastrointestinal tumors with high B7-H6 expression rates (e.g., gastric cancer, colorectal cancer). Additionally, chimeric antigen receptor T cell therapies targeting B7-H6 (e.g., CAR-T and CAR- NK) and monoclonal antibody drugs are under further validation.
| Drug |
Drug Type |
Indication(s) |
Developing Institution |
Highest Phase |
| BI-765049 |
Bispecific T- cell Engager |
Colon cancer, Pancreatic ductal adenocarcinoma, Rectal cancer, Gastric cancer, Head and neck cancer, Liver cancer, Non-small cell lung cancer, Pancreatic cancer, Advanced malignant solid tumor, Metastatic colorectal cancer |
Boehringer Ingelheim GmbH |
Phase I |
| B7-H6 Targeted CAR-T (Adicet) |
CAR-T |
Tumors |
Adicet Bio, Inc. |
Preclinical |
| WO2023104062 |
Monoclonal Antibody |
Infection, Tumors |
Hefei Tian Gang ImmunoPharma Co., Ltd |
Discovery |
| Eneboparatide (AZP-3601) |
Peptide |
Hypoparathyroidism |
Amolyt Pharma |
Phase III |
| WO2023147404 |
CAR-NK |
Immune system diseases, Infection, Tumors |
Rutgers State University of New Jersey |
Discovery |
Table: B7-H6 Drug Development Pipeline (Data Source: Pharmsnap)
4. Future Prospects for B7-H6-targeted Therapy
As a tumor-specific antigen (low expression in normal tissues, high expression in tumors), B7-H6 is an ideal target for cancer immunotherapy. Future efforts must advance its targeted therapy multi-dimensionally:
● Optimize CAR-T/CAR-NK therapies (e.g., utilize CAR-NK to avoid T-cell fratricide; optimize CAR structure to enhance specificity and function).
● Combine with chemotherapy/radiotherapy to induce B7-H6 expression in tumors, thereby enhancing therapeutic sensitivity.
● Deeply dissect its regulatory mechanisms (e.g., role of transcription factors like MYC), the B7-H6/NKp30 axis, and the interaction between membrane-bound/soluble forms and the immune microenvironment.
● Accelerate the clinical translation of novel therapies like BiTEs/ITEs (e.g., trials combining ITE with anti-PD-1). Ultimately propel its transition from preclinical research into efficient, low-toxicity cancer treatment regimens.
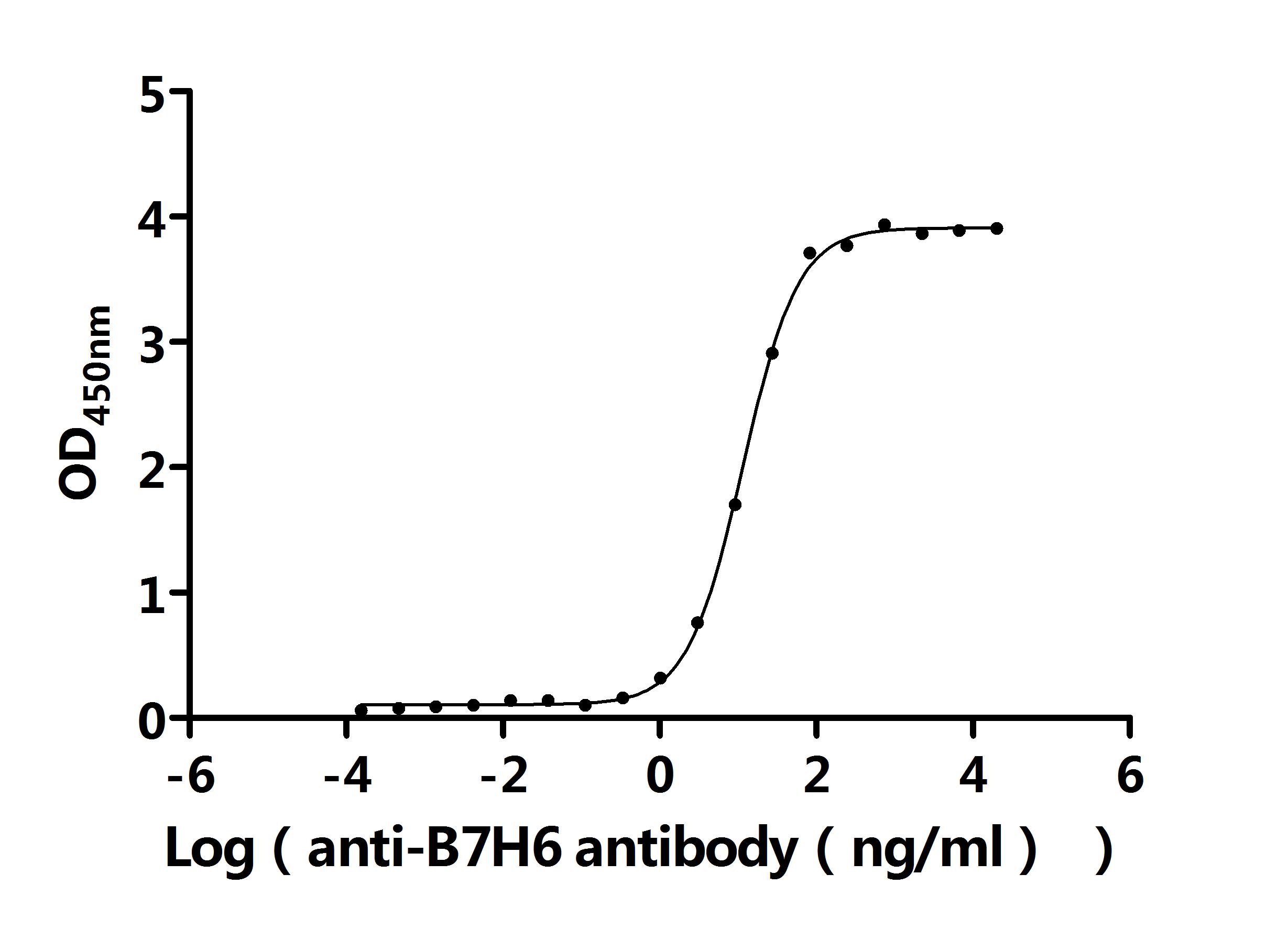
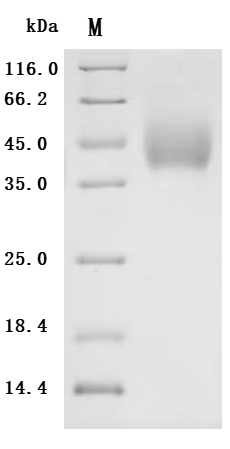
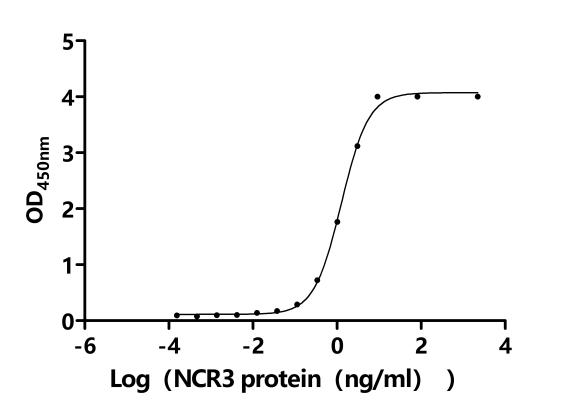
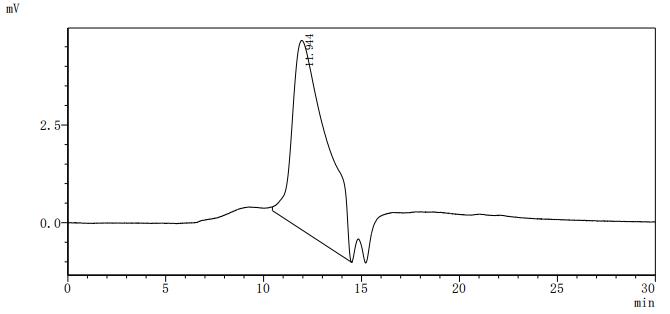
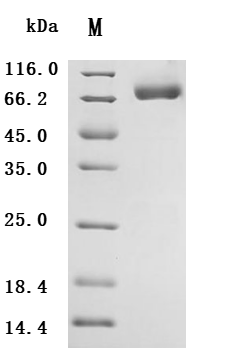
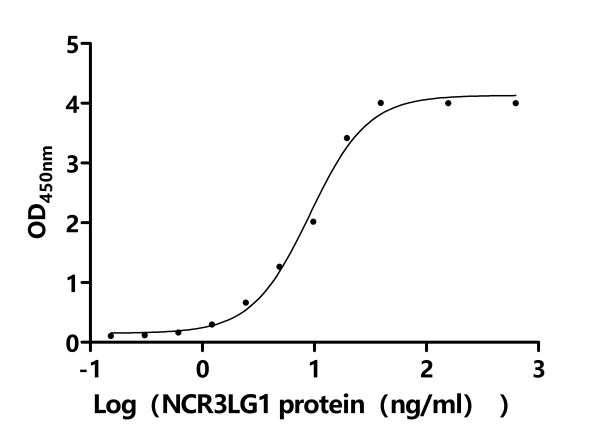
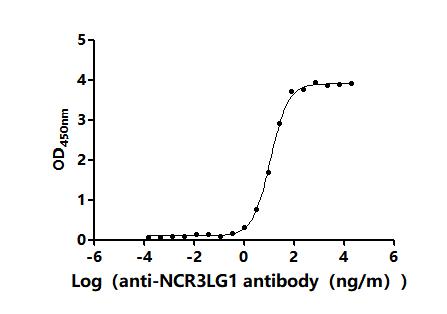
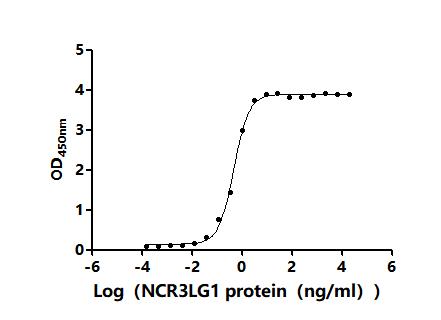
5. CUSABIO's B7-H6 Related Products
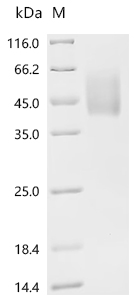
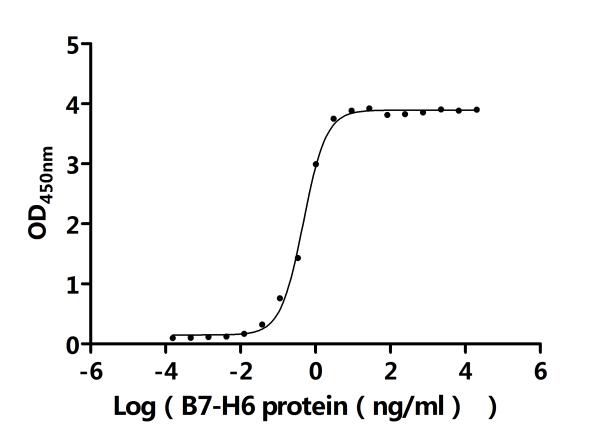
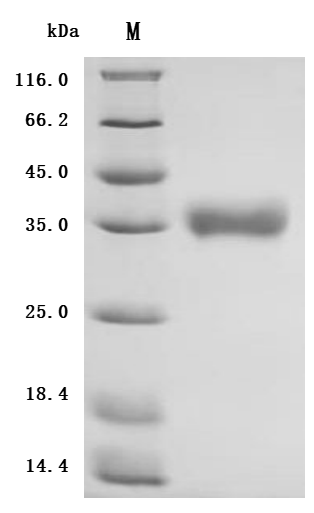
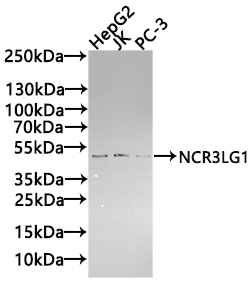
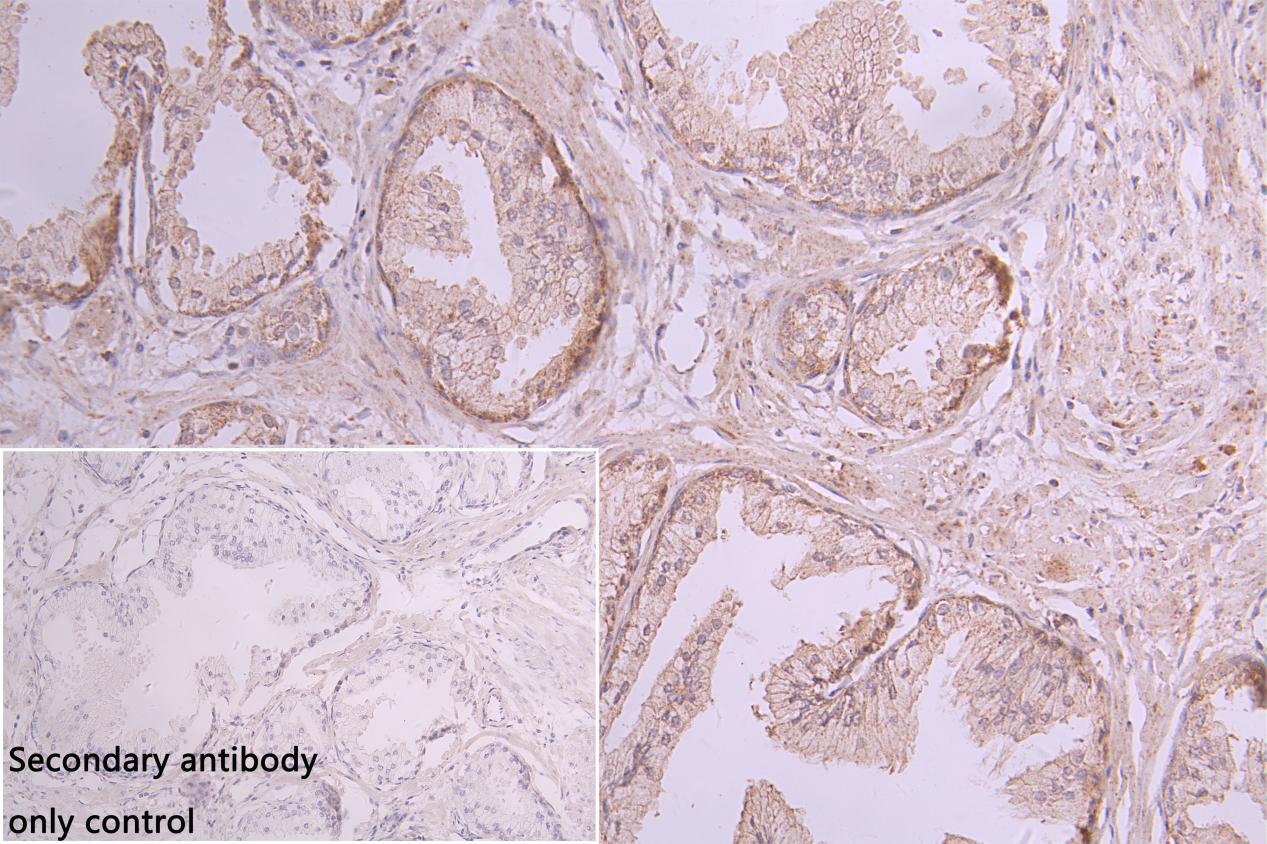
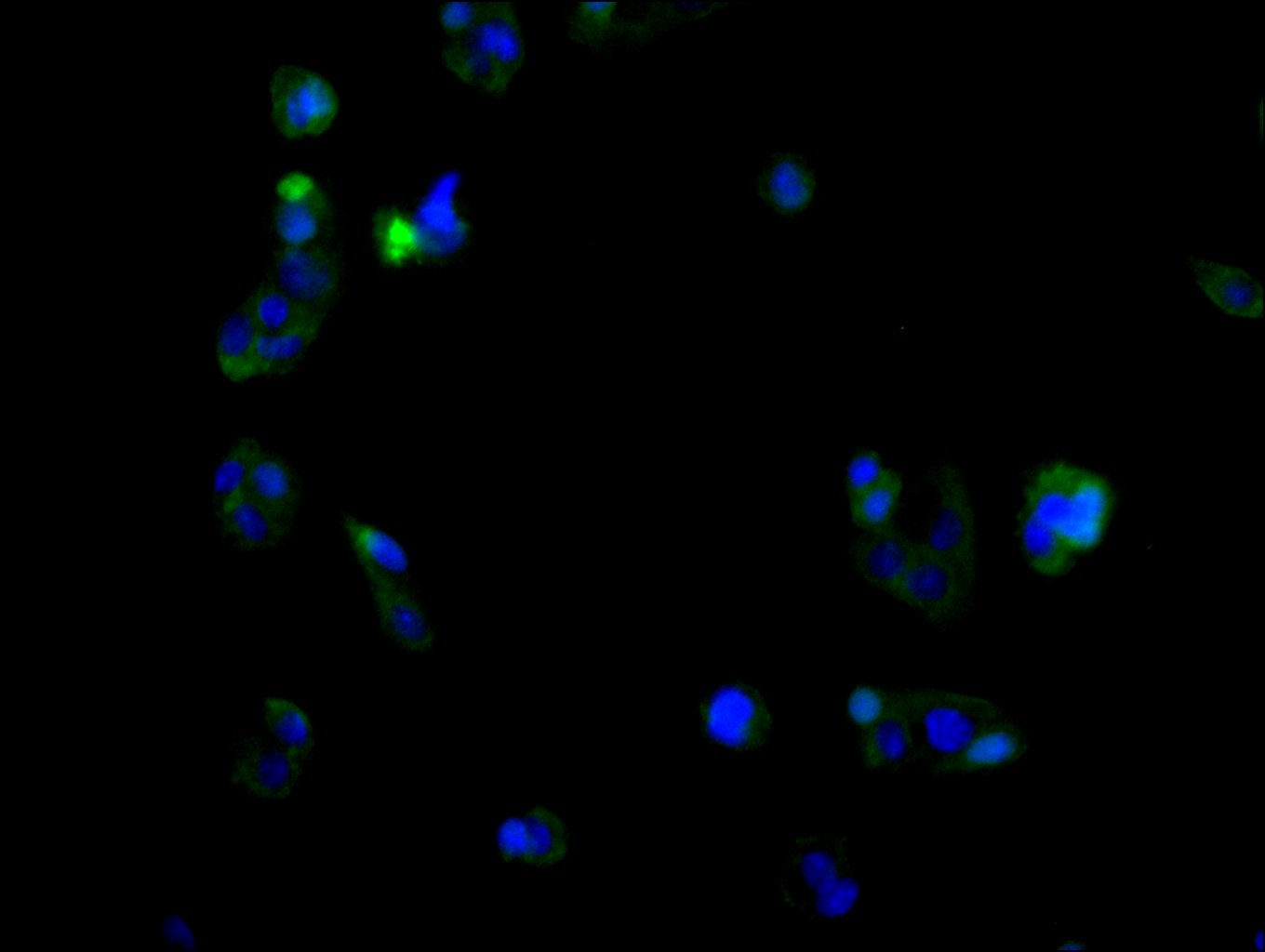
CUSABIO provides high-quality recombinant proteins and antibodies targeting B7-H6 to support mechanistic and translational research:
References
[1] Lee S, Kim JH, Jang IH, et al. Harnessing B7-H6 for Anticancer Immunotherapy: Expression, Pathways, and Therapeutic Strategies. Int J Mol Sci. 2024;25 (19).
[2] Matta J, Baratin M, Chiche L, et al. Induction of B7-H6, a ligand for the natural killer cell-activating receptor NKp30, in inflammatory conditions. Blood. 2013;122 (3):394-404.
[3] Thomas PL, Groves SM, Zhang YK, et al. Beyond Programmed Death-Ligand 1: B7-H6 Emerges as a Potential Immunotherapy Target in SCLC. J Thorac Oncol. 2021;16 (7):1211-1223.
[4] Yuan L, Sun L, Yang S, et al. B7-H6 is a new potential biomarker and therapeutic target of T-lymphoblastic lymphoma. Ann Transl Med. 2021;9 (4):328.
[5] Wu F, Wang J, Ke X. Knockdown of B7-H6 inhibits tumor progression and enhances chemosensitivity in B-cell non-Hodgkin lymphoma. Int J Oncol. 2016;48 (4):1561-70.
[6] Chen L, Feng J, Xu B, et al. B7-H6 expression in human hepatocellular carcinoma and its clinical significance [corrected]. Cancer Cell Int. 2018;18:126.
CUSABIO team. B7-H6: A Key Ligand of NK Cell Immune Checkpoints Reshaping the Landscape of Tumor Immunotherapy. https://www.cusabio.com/c-21241.html




Comments
Leave a Comment