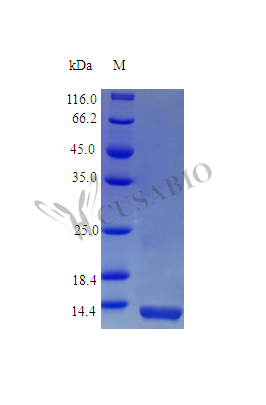
Recombinant Mouse Interleukin-4 protein (Il4) comes from E. coli expression and spans the complete mature protein sequence from amino acids 21-140. The product lacks tags and achieves purity levels exceeding 97%, confirmed through SDS-PAGE analysis. Biological activity remains fully intact, showing an ED50 below 2 ng/ml for murine HT-2 cell proliferation. This translates to specific activity surpassing 5 × 10^5 IU/mg. Endotoxin levels stay controlled under 1.0 EU/µg, which appears to provide adequate reliability for research work.
Interleukin-4 (IL-4) functions as a key cytokine in immune responses. Its primary role involves directing naive helper T cells toward Th2 cell differentiation. The protein appears central to regulating both humoral and adaptive immunity through B cell proliferation and differentiation promotion. Research interest in IL-4 stems largely from its connections to allergic responses and broader immune system regulation.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. T Helper Cell Differentiation Studies
This recombinant mouse IL-4 protein is confirmed to be biologically active (ED₅₀ < 2 ng/ml in murine HT-2 cell proliferation) and suitable for investigating Th2 cell differentiation pathways. The high purity (>97%) and low endotoxin content ensure reliable performance in sensitive immunological assays. Researchers can use this protein to study IL-4-induced transcription factor expression, cytokine production patterns, and epigenetic changes during Th2 polarization with confidence in its functional activity.
2. Cytokine Signaling Pathway Analysis
The biologically active IL-4 protein is appropriate for detailed examination of JAK-STAT6 signaling cascades in mouse cell systems. The defined specific activity (>5×10⁵ IU/mg) enables precise dosing for time-course experiments tracking STAT6 phosphorylation, nuclear translocation, and downstream gene expression. The tag-free design ensures accurate representation of native IL-4 receptor binding kinetics without potential interference from fusion tags.
3. Cell Proliferation and Viability Assays
This protein is validated for proliferation studies, particularly in murine HT-2 cells, making it suitable for similar assays across various mouse immune cell types. The established ED₅₀ value provides a reliable reference for designing concentration gradients in MTT, BrdU incorporation, or other proliferation assays. Researchers can confidently explore IL-4's growth-promoting effects on B cells, T cells, and other immune cell subsets.
4. Antibody Development and Validation
The high-purity, biologically active IL-4 protein serves as an excellent antigen for generating and validating anti-IL-4 antibodies. The confirmed biological activity ensures antibodies will recognize functional epitopes, making it suitable for neutralization assays. The protein can be used in immunization protocols, ELISA development, Western blot validation, and other immunoassays with minimal risk of non-specific reactivity.
5. Comparative Species and Isoform Studies
This E. coli-expressed IL-4 is valuable for comparative studies with IL-4 from other species or expression systems. However, researchers should note that the lack of post-translational modifications in the E. coli-expressed protein may result in functional differences compared to mammalian-expressed IL-4. Comparative binding affinity and functional assessments should account for potential differences due to expression systems rather than inherent protein properties.
Final Recommendation & Action Plan
This E. coli-expressed mouse IL-4 is a high-quality reagent with validated biological activity, making it suitable for all proposed applications. For immediate use, employ it in T helper cell differentiation studies and proliferation assays using the established ED₅₀ as a starting point for dose optimization. The tag-free design ensures authentic receptor binding and signaling studies. When developing antibodies, this protein is ideal for immunization and functional validation. For comparative studies, note that the E. coli expression system produces a non-glycosylated form of IL-4, which may exhibit differences in stability or function compared to native mammalian IL-4; therefore, critical findings should be validated with mammalian-expressed IL-4 when studying PTM-dependent effects. The high purity and low endotoxin levels make this protein particularly valuable for sensitive cell-based assays where contaminant interference must be minimized.