Lately, there is robust evidence that mucins play a crucial role in tumor formation, cell adhesion, immune response, and cell signaling. Typically, several mucins have become hot targets for tumor immunotherapy, such as MUC1, MUC16. Intriguingly, according to ClinicalTrials data, a new study based on MUC17 target (AMG199) is currently being investigated in a phase I trial (NCT04117958), evaluating AMG 199 in patients with MUC17-positive gastric and gastroesophageal junction cancer (G/GEJ). This is the first clinical trial to identify MUC17 as a potential anti-tumor target.
In various tumors, mucins are often aberrantly expressed and are associated with tumor infiltration, metastasis, and prognosis. Compared with other popular mucin targets, MUC17, as an unexpected novel target of mucin family, although not well documented, it is widely thought that MUC17 is a high potential target for tumor immune therapy! It may provide new insights into the treatment of gastric cancer and gastroesophageal junction (G/GEJ) or other diseases!
1. What are Mucins?
Mucins (MUC) are high-molecular-weight glycoprotein that are produced by various epithelial cells. Mucins are the major macromolecular components of mucus. To date, 22 Mucin members have been identified. The mucins are classified into membrane-bound mucins: MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC14, MUC15, MUC17, MUC16, MUC17, MUC17, MUC20, MUC21, MUC22; secreted mucins: MUC2, MUC5AC, MUC5B, MUC6, MUC7, MUC8, MUC9, MUC19 [1-3]. Secreted mucins form a viscous gel that functions as a protective layer over the epithelium and can trap particles and microbes. Membrane-bound mucins form rod-like structures through O-glycosylated tandem repeat sequences in their extracellular domains to build a protective mucus layer [3]. Many inflammatory cytokines, such as IL-1β, IL-4, IL-6, TGF-β, IL-9, IL-13, IFN-γ, TNF-α , have been found to mediate mucin expression in epithelial cells in vitro [4-5].
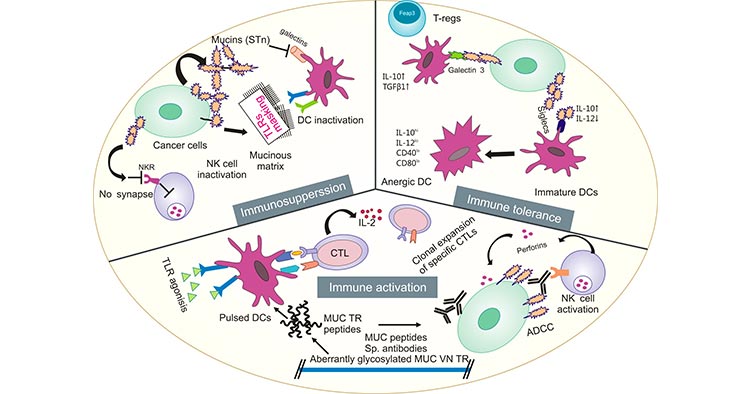
Mucins are widely present on the mucosal surfaces that lines the body's organs and tissues, such as the digestive tract, respiratory tract, and epithelial cells of the liver, pancreas, and kidney [3-4]. Some studies suggested that mucins can be used as a potential tool to predict COVID-19 susceptibility [5]. Notably, many findings revealed that certain mucins engages diverse signaling pathways linked to transformation and tumour progression. Therefore, ongoing work is anticipated to explore the usefulness of mucins in cancer diagnosis and prognosis, even as a potential target for cancer treatment (Figure 1) [6-10].
Figure 1. Mucins play an important role in tumor development [6]
2. What is MUC17?
2.1 MUC17 Structure
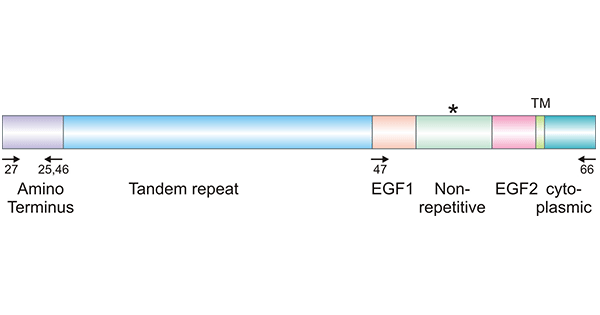
Mucin-17(MUC17, also known as MUC3) is a transmembrane mucin encoded by the MUC17 gene located at the 7q22 region. Mucins are structurally diverse but share similar structural features. The structure of the MUC17 protein harbours a signal peptide, a large tandemly repeated central domain (TR), two epidermal growth factor (EGF)-like domains, a SEA domain, a transmembrane domain (TM) and an 80 amino acid cytoplasmic tail. The long Nterminal extracellular domain of MUC17 can potentially affect cell-to-cell adhesion by altering the interaction of cell adhesion molecules and is a site of extensive glycosylation. The cytoplasmic domain has many predicted phosphorylation sites that may mediate signal transduction. The extracellular domain determines the MUC-specific spatial structure and immunogenicity. The roles of the two EGF-like domains are not completely understood (Figure 2) [11-12, 14].
Figure 2. MUC17 structure [14]
2.2 MUC17 Expression
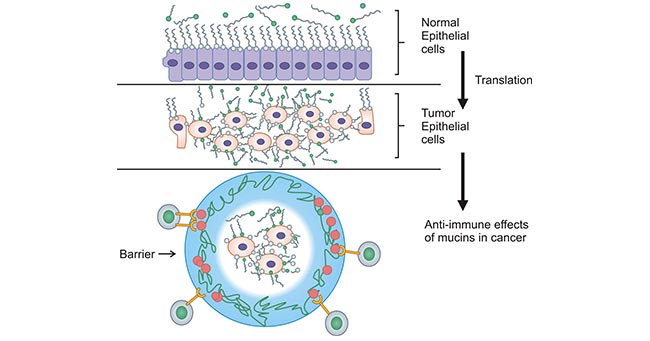
MUC17 is mainly expressed in the intestine and is a major component of intestinal mucus. MUC17 expression enhances the intestinal mucus barrier and dampening inflammation at damaged mucosal sites [11]. More importantly, MUC17 is overexpressed in about half of gastric cancers. Generally, mucins are expressed at the surface of densely packed columnar cells in normal epithelium which form of protective and lubricating function on the mucosal cell layer. However, in malignant tumor cells, mucins are highly expressed on the transformed tumor cells and cover all surface areas of the cell membrane, and the tumor cells become rounded in shape, increase cell-cell porosity and lose polarity (Figure 3) [42].
Figure 3. Expression of mucins on normal versus tumor epithelial cells [42]
2.3 MUC17 Function
Functional characterization of MUC17 in certain cancer have been observed. It has been reported that MUC17 was more highly expressed in gastric cancer specimens, with favourable prognosis for patients. Further results demonstrate that MUC17 as a gastric cancer suppressor protein has the therapeutic potential for human GC [20, 22]. In addition, loss of punctate immunostaining pattern of MUC17 was associated with an aggressive behavior of colorectal adenocarcinoma and shortened patient survival [41]. In the absence of MUC17, increased tumor weights were observed in pancreatic adenocarcinoma cells [42]. These results implicated that MUC17 may act as tumour-suppressing genes. Further research is needed to determine the specific role of MUC17 in the pathogenesis of these cancers.
3. MUC17-Mediated Signal Transduction Pathway
As a membrane-bound mucin with important biological functions, MUC17 expression levels are associated with the proliferation of various inflammatory and tumor cells, which provides important clues for the prevention and treatment of MUC17-associated diseases, but its molecular mechanism remains obscure.
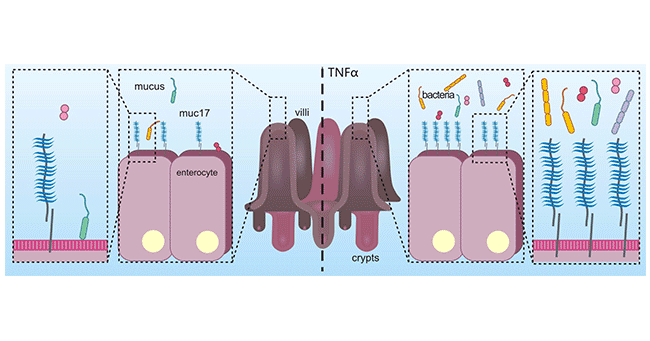
In colon adenocarcinoma cells, long-term stimulation with the pro-inflammatory cytokine TNFα, which induces an inflammatory state, can lead to an increase in MUC17, which in turn protects colon adenocarcinoma cells from adhesion by enteropathogenic E. coli. The results suggest that MUC17 can play a defensive role in inflammation (Figure 4) [21]. Another study suggested that the EGF-like domain of MUC17 activated Rho/Rock pathway through interacting MYH9, and then inhibited NFκB pathway, which stimulated by inflammation factors. The observations indicated that MUC17 could inhibit the progression of human gastric cancer by limiting inflammatory responses through a MYH9-p53-RhoA regulatory feedback loop [22].
Other reports on MUC17 reveal that the C-terminus of MUC17 binds to scaffolding proteins PDZK1, which stabilizes its localization to the enterocyte apical membrane of the small intestine [23]; DNA methylation and histone H3K9 modifications contribute to MUC17 expression [24]. Also in colon cancer, a study suggested that MUC17 can bind to the EGFR family of ERBB2, activating Wnt signaling, regulating EMT process [25-26].
In addition, it has been shown that colostrum-derived, synthetic peptide containing the TFLK motif stimulates increased expression of MUC17 in colon cell line HT29 cells. The TFLK motif is a characteristic structural sequence of the MSAA3 protein, which is important for the prevention of pathogenic bacterial attack and protection of the gastrointestinal tract in neonates. This mechanism will provide new ideas for enhancing the intestinal mucus barrier and preventing multiple inflammatory diseases [27-28].
Figure 4. MUC17 plays a defensive role in inflammation [21]
4. The Roles of MUC17 in Cancers
As a transmembrane mucin, MUC17 plays an important role in defense against bacterial attack. Deregulated MUC17 production has been associated with various types of cancer and inflammatory disorders [29, 30]. Currently, a large number of studies have focused on the association of MUC17 with gastrointestinal tract tumors and other tumors such as colon, gastric, pancreatic, esophageal, and breast cancers [31-40].
In colorectal cancer, based on the immunohistochemical analysis, researchers observed uniform MUC17 staining in the cytoplasm in all benign (normal) colonic epithelial tissues. Whereas in CRCs, there are only punctate staining (60 of 136, 44%), a complete lack of staining (null) or diffuse low levels of MUC17 (76 of 136, 56%). Loss of punctate immunostaining pattern of MUC17 was associated with an aggressive behavior of CRCs and shortened patient survival. In vitro cell based analyses have suggested that cells with stable inhibition of MUC17 by siRNA had significantly decreased cell cycle arrest in G0/G1 or S phase. Collectively, a series of the cell-based experiments suggested that MUC17 may be the protein product of a potential tumor suppressor gene in colorectal cancer [16, 31, 41].
In pancreatic cancer, MUC17 was overexpressed in pancreatic cancer tissues compared with normal pancreatic tissues. Detection of pancreatic ductal adenocarcinoma tissues by immunohistochemistry revealed significantly enhanced MUC17 expression, suggesting that MUC17 could be one of the independent prognostic factors [32-33]. Another study suggested that changes in MUC17 expression were positively correlated with the development of Barrett's esophagus, esophageal adenocarcinoma, pancreatic intestinalization and pancreatic cancer [35-37]. In breast cancer, MUC17 knockdown was found to be associated with drug sensitivity in patients, further revealing that the activity of MUC17 and PCNX1 could influence chemotherapy resistance, like making tumor cells more sensitive to the drugs. Namely, MUC17 could be potential biomarkers of chemotherapy response in breast cancer [25, 38-39].
In gastric cancer and gastroesophageal junction cancer (G/GEJ), it has been demonstrated that MUC17 is overexpressed on G/GEJ cell membranes. Thereby, MUC17 is considered as a tumor-associated antigen (TAA). Gastric cancer is associated with gastric mucosal barrier dysfunction. Damage to the gastric mucosal barrier usually leads to chronic inflammation, which is a major factor in gastric carcinogenesis. Further studies indicated that when MUC17 expression was reduced, the clonogenic ability was diminished. Besides, the number of tumor-invasive cells were decreased, as well as the cell migration and cell proliferation. MUC17 Knockdown restrained the invasive and proliferative ability of gastric cancer cells, suggesting that MUC17 might be new target for gastric cancer therapy [40].
5. MUC17 Clinical Research Prospects
A bispecific antibody targeting MUC17 (AMG199; MUC17 x CD3) from Amgen Inc. has been investigated in I clinical trial, which is designed for the treatment in MUC17-positive solid tumors, including gastrointestinal, gastroesophageal junction, colorectal and pancreatic cancers. In 2020, Amgen Inc. received the FDA granted orphan drug designation to AMG199. At the 2021 ASCO annual meeting, Amgen announced the first Phase I open-label dose-escalation study in humans to evaluate the dose-limiting toxicity and efficacy of AMG199 in patients with MUC17-positive G/GEJ and to determine the maximum tolerated dose (MTD) and/or recommended Phase 2 dose (RP2D). It is stated that the phase I studies will be completed in March, 2024.
Many works suggest that mucins are overexpressed in different malignant tumors. The roles of mucins in tumors provide important clues for the design of mucin-based antitumor drugs. In this regard, certain mucins function as potential targets for therapeutic interventions in different cancers. Meanwhile, with further works on the structure and function of MUC17, MUC17 has also emerged as a highly attractive target for the cancer treatment. It is expected that MUC17 and the other transmembrane mucins will also be identified as direct drug targets with therapeutic promise.
To assist researchers or pharmaceutical companies in their research on MUC17 in tumor and inflammatory diseases, CUSABIO offers the MUC17 active protein product (Code: CSB-MP727848HU) to support your research on the mechanism of MUC17 or its potential clinical value.
Recombinant Human Mucin-17(MUC17), partial (Active)
High Purity Validated by SDS-PAGE
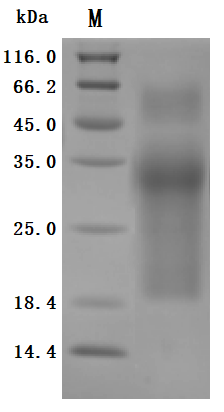
The purity was greater than 95% as determined by SDS-PAGE.(Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
Excellent Bioactivity Validated by Functional ELISA
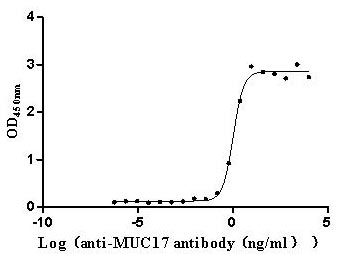
Immobilized Human MUC17 at 2 μg/mL can bind Anti-MUC17 recombinant antibody (CSB-RA727848MA1HU), the EC50 is 0.9057-1.259 ng/mL.
References
[1] Dekker, Jan, et al. "The MUC family: an obituary." Trends in biochemical sciences 27.3 (2002): 126-131.
[2] Alcântara, Angélica Leite de, et al. "MUC family influence on acute lymphoblastic leukemia in Native American populations from Brazilian Amazon." (2022): e19025-e19025.
[3] Sousa, Andreia M., et al. "Reflections on MUC 1 glycoprotein: the hidden potential of isoforms in carcinogenesis." apmis 124.11 (2016): 913-924.
[4] Długosz, Ewa, et al. "Toxocara canis mucins among other excretory-secretory antigens induce in vitro secretion of cytokines by mouse splenocytes." Parasitology research 114.9 (2015): 3365-3371.
[5] Lin, Susanne Je-Han, Bailey Arruda, and Eric Burrough. "Alteration of Colonic Mucin Composition and Cytokine Expression in Acute Swine Dysentery." Veterinary Pathology 58.3 (2021): 531-541.
[6] Bhatia R, Gautam SK, Cannon A, et al. Cancer-associated mucins: role in immune modulation and metastasis. Cancer Metastasis Rev. 2019;38(1-2):223-236.
[7] Brockhausen, Inka, and Jacob Melamed. "Mucins as anti-cancer targets: Perspectives of the glycobiologist." Glycoconjugate Journal 38.4 (2021): 459-474.
[8] Marimuthu, Saravanakumar, et al. "Mucins reprogram stemness, metabolism and promote chemoresistance during cancer progression." Cancer and Metastasis Reviews 40.2 (2021): 575-588.
[9] Wi, Dong-Han, Jong-Ho Cha, and Youn-Sang Jung. "Mucin in cancer: a stealth cloak for cancer cells." BMB reports 54.7 (2021): 344.
[10] Kufe, Donald W. "Mucins in cancer: function, prognosis and therapy." Nature Reviews Cancer 9.12 (2009): 874-885.
[11] Shekels LL, Ho SB. Characterization of the mouse Muc3 membrane bound intestinal mucin 5' coding and promoter regions: regulation by inflammatory cytokines. Biochim Biophys Acta. 2003;1627(2-3):90-100.
[12] Desseyn, Jean-Luc, Daniel Tetaert, and Valérie Gouyer. "Architecture of the large membrane-bound mucins." Gene 410.2 (2008): 215-222.
[13] Gum Jr, James R., et al. "MUC17, a novel membrane-tethered mucin." biochemical and biophysical research communications 291.3 (2002): 466-475.
[14] Moniaux, Nicolas, et al. "Characterization of human mucin MUC17: Complete coding sequence and organization." Journal of Biological Chemistry 281.33 (2006): 23676-23685.
[15] Schneider, Hannah, et al. "The human transmembrane mucin MUC17 responds to TNFα by increased presentation at the plasma membrane." Biochemical Journal 476.16 (2019): 2281-2295.
[16] Senapati, Shantibhusan, et al. "Expression of intestinal MUC17 membrane-bound mucin in inflammatory and neoplastic diseases of the colon." journal of clinical pathology 63.8 (2010): 702-707.
[17] Gál, Eleonóra, et al. "Importance of MUC17 in the Bile-Induced Pancreatic Cancer Progression."(2022).
[18] Layunta, Elena, et al. "IL-22 promotes the formation of a MUC17 glycocalyx barrier in the postnatal small intestine during weaning." Cell Reports 34.7 (2021): 108757.
[19] Bailis, Julie M., et al. "Preclinical evaluation of BiTE® immune therapy targeting MUC17 or CLDN18. 2 for gastric cancer." Cancer Research 80.16_Supplement (2020): 3364-3364.
[20] Lordick, F., et al. "P-76 A phase 1 study of AMG 199, a half-life extended bispecific T-cell engager (HLE BiTE®) immune therapy, targeting MUC17 in patients with gastric and gastroesophageal junction cancer." Annals of Oncology 31 (2020): S114.
[21] Schneider, Hannah, et al. "The human transmembrane mucin MUC17 responds to TNFα by increased presentation at the plasma membrane." Biochemical Journal 476.16 (2019): 2281-2295.
[22] Yang, Bing, et al. "Mucin 17 inhibits the progression of human gastric cancer by limiting inflammatory responses through a MYH9-p53-RhoA regulatory feedback loop." Journal of Experimental & Clinical Cancer Research 38.1 (2019): 1-13.
[23] Malmberg, Emily K., et al. "The C-terminus of the transmembrane mucin MUC17 binds to the scaffold protein PDZK1 that stably localizes it to the enterocyte apical membrane in the small intestine." Biochemical Journal 410.2 (2008): 283-289.
[24] Kitamoto, Sho, et al. "DNA methylation and histone H3-K9 modifications contribute to MUC17 expression." Glycobiology 21.2 (2011): 247-256.
[25] Yu, Kuan, et al. "Intratumoral PD-1+ CD8+ T cells associate poor clinical outcomes and adjuvant chemotherapeutic benefit in gastric cancer." British journal of cancer 127.9 (2022): 1709-1717.
[26] Al Amri, Waleed S., et al. "Genomic and Expression Analyses Define MUC17 and PCNX1 as Predictors of Chemotherapy Response in Breast CancerMUC17 and PCNX1 Predict Chemoresponse." molecular cancer therapeutics 19.3 (2020): 945-955.
[27] Pan, Qiong, et al. "Enhanced Membrane-tethered Mucin 3 (MUC3) Expression by a Tetrameric Branched Peptide with a Conserved TFLK Motif Inhibits Bacteria Adherence*[S]." Journal of Biological Chemistry 288.8 (2013): 5407-5416.
[28] Qiong, P. A. N., et al. "Influence of MSAA3 protein fragment and modified peptide thereof on the MUC3 expression of HT29 cells." Medical Journal of Chinese People's Liberation Army 36.10 (2011): 1062-1064.
[29] Resta-Lenert, Silvia, et al. "Muc17 protects intestinal epithelial cells from enteroinvasive E. coli infection by promoting epithelial barrier integrity. "American Journal of Physiology-Gastrointestinal and Liver Physiology 300.6 (2011): G1144-G1155.
[30] Yang, Ching-Wen, et al. "Genetic variations of MUC17 are associated with endometriosis development and related infertility." BMC medical genetics 16.1 (2015): 1-7.
[31] Jiang, Zhipeng, et al. "Analysis of TGCA data reveals genetic and epigenetic changes and biological function of MUC family genes in colorectal cancer." future Oncology 15.35 (2019): 4031-4043.
[32] Kitamoto, Sho, et al. "Expression of MUC17 is regulated by HIF1α-mediated hypoxic responses and requires a methylation-free hypoxia responsible element in pancreatic cancer." (2012): e44108.
[33] Kitamoto, Sho, et al. "Expression of MUC17, a marker of pancreatic cancer, is under the control of epigenetic modifications." Cancer Research 71.8_Supplement (2011): 82-82.
[34] Hyland, Paula L., et al. "Global changes in gene expression of Barrett's esophagus compared to normal squamous esophagus and gastric cardia tissues." PLoS One 9.4 (2014): e93219.
[35] Ye et al. "The role of MUC1, MUC3 and MUC6 in Barrett's esophagus, esophageal adenocarcinoma, cardia intestinal metaplasia and cardia adenocarcinoma." 2006.
[36] Glickman, Jonathan N., Aliakbar Shahsafaei, and Robert D. Odze. "Mucin core peptide expression can help differentiate Barrett's esophagus from intestinal metaplasia of the stomach." The American journal of surgical pathology 27.10 (2003): 1357-1365.
[37] Lonie, James M., Andrew P. Barbour, and Riccardo Dolcetti. "Understanding the immuno-biology of oesophageal adenocarcinoma: Towards improved therapeutic approaches. "Cancer Treatment Reviews 98 (2021): 102219.
[38] Rakha, Emad A., et al. "Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer." Modern Pathology 18.10 (2005): 1295-1304.
[39] Al Amri, W., et al. "Abstract P3-06-19: MUC17 and PCNX1 as mediators of chemotherapy response in breast cancer." Cancer Research 79.4_Supplement (2019): P3-06.
[40] Chao, Joseph, et al. "Trial in progress: a phase I study of AMG 199, a half-life extended bispecific T-cell engager (HLE BiTE) immune therapy, targeting MUC17 in patients with gastric and gastroesophageal junction (G/GEJ) cancer." (2020): TPS4649-TPS4649.
[41] Katkoori, Venkat, et al. "Abstract# 4362: MUC17 is a potential tumor suppressor gene in colorectal adenocarcinoma." Cancer Research 69.9_Supplement (2009): 4362-4362.
[42] Lin, Bo, et al. "Alpha-fetoprotein binding mucin and scavenger receptors: an available bio-target for treating cancer." Frontiers in Oncology 11 (2021): 625936.
CUSABIO team. Mucin-17/MUC17: An Unexpected Target for Tumor Immune Therapy, Notably Gastric Cancer!. https://www.cusabio.com/c-21101.html






Comments
Leave a Comment