[1] Germano G, Frapolli R, Belgiovine C, et al. Role of Macrophage Targeting in the Antitumor Activity of Trabectedin [J]. Cancer cell, 2013, 23(2): 249-262.
[2] Mosser D M, Edwards J P. Exploring the full spectrum of macrophage activation [J]. NATURE REVIEWS IMMUNOLOGY, 2008, 8(12): 958-969.
[3] Abramson S L, Gallin J I. IL-4 inhibits superoxide production by human mononuclear phagocytes [J]. Journal of Immunology, 1990, 144(2): 625-630.
[4] Biswas S K, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm [J]. Nature Immunology, 2010, 11(10): 889-896.
[5] Grohmann U, Belladonna M L, Vacca C, et al. Positive Regulatory Role of IL-12 in Macrophages and Modulation by IFN-γ [J]. The Journal of Immunology, 2001, 167(1): 221-227.
[6] Ginderachter J A V, Movahedi K, Ghassabeh G H, et al. Classical and alternative activation of mononuclear phagocytes: Picking the best of both worlds for tumor promotion [J]. 2006, 211(6-8): 0-501.
[7] Wynn T A, Chawla A, Pollard J W. Macrophage biology in development, homeostasis and disease [J]. Nature, 2013, 496(7446): 445-455.
[8] Xu J, Chi F, Tsukamoto H. Notch signaling and M1 macrophage activation in obesity-alcohol synergism [J]. Clinics and Research in Hepatology and Gastroenterology, 2015, 39: S24-S28.
[9] Saliba D, Heger A, Eames H, et al. IRF5: RelA Interaction Targets Inflammatory Genes in Macrophages [J]. Cell Reports, 2014, 8(5): 1308-1317.
[10] Lu G, Zhang R, Geng S, et al. Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization [J]. Nature Communications, 2015, 6: 6676.
[11] Hall C J, Boyle R H, Astin J W, et al. Immunoresponsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating β-oxidation-dependent mitochondrial ROS production [J]. Cell Metabolism, 2013, 18(2): 265-278.
[12] Zizzo G, Cohen P L. IL-17 Stimulates Differentiation of Human Anti-Inflammatory Macrophages and Phagocytosis of Apoptotic Neutrophils in Response to IL-10 and Glucocorticoids [J]. The Journal of Immunology, 2013, 190(10): 5237-5246.
[13] Arranz A, Doxaki C, Vergadi E, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization [J]. Proceedings of the National Academy of Sciences, 2012, 109(24): 9517-9522.
[14] Xu F, Kang Y, Zhang H, et al. Akt1-Mediated Regulation of Macrophage Polarization in a Murine Model of Staphylococcus aureus Pulmonary Infection [J]. Journal of Infectious Diseases, 2013, 208(3): 528-538.
[15] Macgarvey N C, Suliman H B, Bartz R R, et al. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis [J]. American Journal of Respiratory & Critical Care Medicine, 2012, 185(8): 851-61.
[16] Tedescoa S, Bolegoa C, Tonioloa A, et al. Phenotypic activation and pharmacological outcomes of spontaneously differentiated human monocyte-derived macrophages [J]. Immunobiology, 2015, 220(5): 545-554.
[17] Rebelo S P, Pinto C, Martins T R, et al. 3D-3-culture: A tool to unveil macrophage plasticity in the tumour microenvironment [J]. Biomaterials, 2018.
[18] Wang S, Zhang J, Sui L, et al. Antibiotics induce polarization of pleural macrophages to M2-like phenotype in patients with tuberculous pleuritis [J]. Scientific Reports, 2017, 7(1).
[19] Shabo I, Svanvik J. Expression of macrophage antigens by tumor cells [J]. Oxygen Transport to Tissue XXXIII, 2011, 714: 141-150.
[20] Stoermer K A, Burrack A, Oko L, et al. Genetic Ablation of Arginase 1 in Macrophages and Neutrophils Enhances Clearance of an Arthritogenic Alphavirus [J]. The Journal of Immunology, 2012, 189(8): 4047-4059.
[21] Raes G, Baetselier P D, Wim No?l, et al. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages [J]. Journal of Leukocyte Biology, 2002, 71(4): 597.
[22] Dragomir A C D, Sun R, Choi H, et al. Role of Galectin-3 in Classical and Alternative Macrophage Activation in the Liver following Acetaminophen Intoxication [J]. The Journal of Immunology, 2012, 189(12): 5934-5941.
[23] Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity [J]. NATURE REVIEWS IMMUNOLOGY, 2011, 11(11): 750-761.
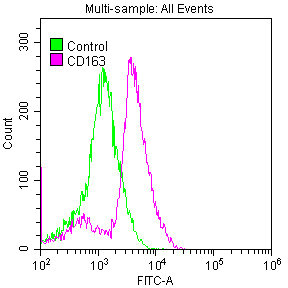
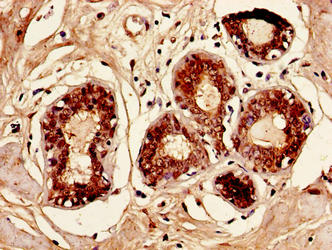
[24] Shabo I, Olsson H, Elkarim R, et al. Macrophage Infiltration in Tumor Stroma is Related to Tumor Cell Expression of CD163 in Colorectal Cancer [J]. Cancer Microenvironment, 2014, 7(1-2): 61-69.
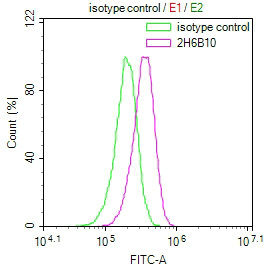
[25] Sanyal R, Polyak M J, Zuccolo J, et al. MS4A4A: a novel cell surface marker for M2 macrophages and plasma cells [J]. Immunology and Cell Biology, 2017.
[26] Jablonski K A, Amici S A, Webb L M, et al. Novel Markers to Delineate Murine M1 and M2 Macrophages [J]. PLOS ONE, 2015, 10(12): e0145342-.

-AC1.jpg)
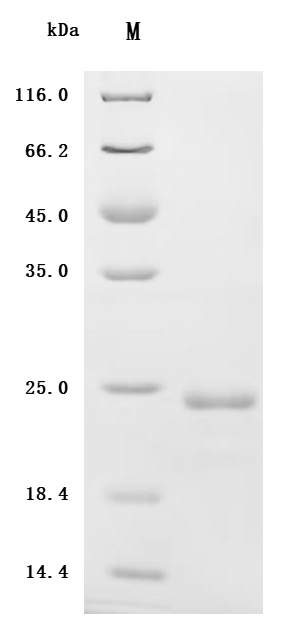
-SDS.jpg)



Comments
Leave a Comment