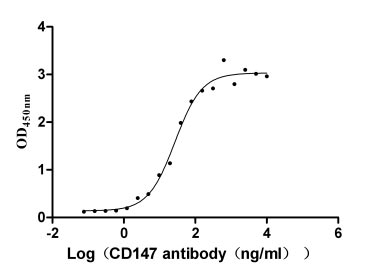
This recombinant protein is a mammalian cell-expressed, C-terminal hFc-tagged form of human Basigin (BSG/CD147), encompassing the amino acids 22-205 of the human BSG. It exhibits high purity (>95% by SDS-PAGE) and low endotoxin levels (<1.0 EU/μg, LAL method), ensuring suitability for sensitive cellular and biochemical assays. Functional validation via ELISA demonstrates its binding activity to anti-CD147 recombinant antibodies, with an EC50 range of 21.95–33.12 ng/mL. The hFc tag facilitates purification and detection while maintaining structural integrity. Provided as a lyophilized powder, this recombinant human BSG protein is optimized for stability and reconstitution in downstream applications. Mammalian expression ensures proper post-translational modifications, making it a critical tool for studying BSG's roles in cellular signaling, immune regulation, and interactions with ligands such as cyclophilins or monocarboxylate transporters.
Human Basigin (BSG), also known as CD147/EMMPRIN, is a significant transmembrane glycoprotein that plays diverse roles in cellular processes. It is crucial within various biological contexts, including immune responses, tissue physiology, and cancer biology. Basigin is particularly noteworthy for its involvement in mediating the transport of monocarboxylic acids, which are vital for cellular metabolism and communication.
The human basigin gene is located on chromosome 19 and has multiple isoforms, specifically four known variants (BSG-1, BSG-2, BSG-3, and BSG-4) generated by alternate splicing [1]. Each of these isoforms has distinct functional implications. For instance, BSG-2 has been identified as a receptor that binds ligand proteins, influencing cell communication and signaling mechanisms that may affect processes such as inflammation and tumorigenesis [2]. Furthermore, basigin is not only expressed in many tissues but also has been extensively studied concerning its role in cancers, particularly through the modulation of matrix metalloproteinases (MMPs), which are involved in the degradation of the extracellular matrix [1][3].
Basigin's interaction with pathogens highlights its significance beyond normal physiological roles. Notably, it is a crucial receptor for the Plasmodium falciparum RH5 protein, facilitating the entry of malaria parasites into human erythrocytes. This interaction emphasizes the relevance of basigin in host-pathogen dynamics and suggests its potential as a therapeutic target [4][5]. Research has shown that targeting basigin can disrupt the parasite's ability to invade red blood cells, presenting a novel avenue for malaria treatment [6]. Additionally, basigin has been implicated in immune system functions. It modulates the activation of T lymphocytes and the secretion of matrix metalloproteinase-9, further illustrating its role in both cancer and autoimmune responses [7].
References:
[1] I. Marchiq, J. Albrengues, S. Granja, C. Gaggioli, J. Pouysségur, & M. Simon. Knock out of the basigin/cd147 chaperone of lactate/h+ symporters disproves its pro-tumour actionviaextracellular matrix metalloproteases (mmps) induction. Oncotarget, vol. 6, no. 28, p. 24636-24648, 2015. https://doi.org/10.18632/oncotarget.4323
[2] R. Belton, L. Chen, F. Mesquita, & R. Nowak. Basigin-2 is a cell surface receptor for soluble basigin ligand. Journal of Biological Chemistry, vol. 283, no. 26, p. 17805-17814, 2008. https://doi.org/10.1074/jbc.m801876200
[3] T. Kanekura, X. Chen, & T. Kanzaki. Basigin (cd147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. International Journal of Cancer, vol. 99, no. 4, p. 520-528, 2002. https://doi.org/10.1002/ijc.10390
[4] L. Chen, Y. Xu, et al. Crystal structure of pfrh5, an essential p. falciparum ligand for invasion of human erythrocytes. Elife, vol. 3, 2014. https://doi.org/10.7554/elife.04187
[5] L. Plenderleith, W. Liu, et al., Adaptive evolution of rh5 in ape plasmodium species of the laverania subgenus. Mbio, vol. 9, no. 1, 2018. https://doi.org/10.1128/mbio.02237-17
[6] P. Acharya, M. Garg, P. Kumar, A. Munjal, & K. Raja. Host–parasite interactions in human malaria: clinical implications of basic research. Frontiers in Microbiology, vol. 8, 2017. https://doi.org/10.3389/fmicb.2017.00889
[7] G. Pistol, C. Matache, et al. Roles of cd147 on t lymphocytes activation and mmp‐9 secretion in systemic lupus erythematosus. Journal of Cellular and Molecular Medicine, vol. 11, no. 2, p. 339-348, 2007. https://doi.org/10.1111/j.1582-4934.2007.00022.x