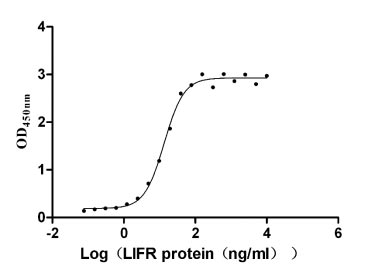
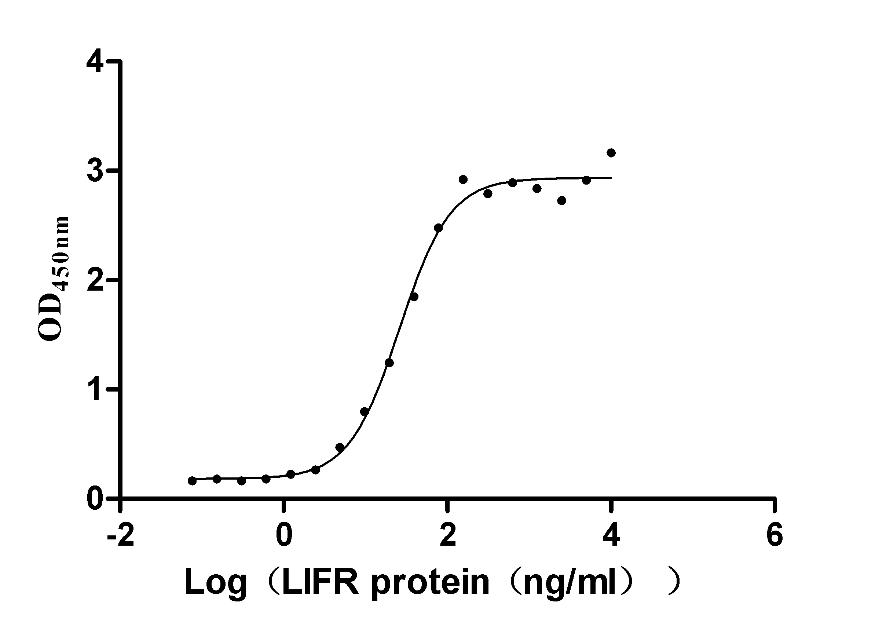
The recombinant human LIFR protein is an active protein produced in mammalian cells. The gene fragment encoding the 45-833aa of the human LIFR is cloned into a plasmid containing a C-terminal hFc-Flag-tag. Transfection of mammalian cells with the recombinant plasmid is followed by the addition of antibiotics after 24 hours to screen the LIFR protein-expressing cells. The selected cells are cultured and induced to express the LIFR proteins. The LIFR protein is extracted from the cell lysate and purified via affinity chromatography. SDS-PAGE determines a purity of this LIFR protein of up to 95%, while the LAL method ensures its endotoxin levels are below 1.0 EU/μg. ELISA demonstrates the binding of this human LIFR to its ligand ILF (CSB-MP012928HU) with an EC50 between 11.68 and 15.52 ng/mL.
The human LIFR is a crucial component of the signaling pathway activated by the leukemia inhibitory factor (LIF), a member of the IL-6 cytokine family. LIFR functions as a receptor that, upon binding with LIF, forms a heterodimer with the glycoprotein gp130. This complex is essential for transducing signals that regulate various biological processes, including cell proliferation, differentiation, and survival, particularly in the context of cancer [1][2].
LIFR does not possess intrinsic tyrosine kinase activity; however, it associates with the JAK family of cytoplasmic tyrosine kinases, which are activated upon LIF binding. This activation leads to the phosphorylation of the STAT3 and other downstream signaling pathways such as MAPK and PI3K/Akt [3][4][5]. These pathways are implicated in various cellular responses, including epithelial-mesenchymal transition (EMT), which is a critical process in cancer metastasis [6][7].
In the context of cancer, LIFR has been identified as a dual-function receptor. It can act as a tumor suppressor in certain contexts while promoting oncogenic processes in others. LIFR has been shown to inhibit cell migration and invasion in breast cancer [8][9]. Conversely, in triple-negative breast cancer, LIFR signaling has been associated with promoting tumor growth and resistance to therapies [3][10].
Moreover, LIFR's involvement in stem cell biology and embryogenesis has been highlighted, as it plays a significant role in maintaining the pluripotency of embryonic stem cells through the activation of the YAP/TAZ-TEAD transcriptional complex, which is regulated by the Hippo signaling pathway [11][5].
References:
[1] D. Chen, Y. Sun, Y. Wei, P. Zhang, A. Rezaeian, J. Teruya‐Feldstein, et al. Lifr is a breast cancer metastasis suppressor upstream of the hippo-yap pathway and a prognostic marker, Nature Medicine, vol. 18, no. 10, p. 1511-1517, 2012. https://doi.org/10.1038/nm.2940
[2] M. Jorgensen and P. Puente,.Leukemia inhibitory factor: an important cytokine in pathologies and cancer, Biomolecules, vol. 12, no. 2, p. 217, 2022. https://doi.org/10.3390/biom12020217
[3] M. Li, S. Viswanadhapalli, B. Santhamma, U. Pratap, Y. Luo, J. Liu, et al. Lifr inhibition enhances the therapeutic efficacy of hdac inhibitors in triple negative breast cancer, Communications Biology, vol. 4, no. 1, 2021. https://doi.org/10.1038/s42003-021-02741-7
[4] C. Giorgio, A. Lupia, S. Marchianò, M. Bordoni, R. Bellini, C. Massa, et al. Repositioning mifepristone as a leukaemia inhibitory factor receptor antagonist for the treatment of pancreatic adenocarcinoma, Cells, vol. 11, no. 21, p. 3482, 2022. https://doi.org/10.3390/cells11213482
[5] A. Catunda, E. Gócza, B. Carstea, L. Hiripi, H. Hayes, C. Rogel-Gaillard, et al. Characterization, chromosomal assignment, and role of lifr in early embryogenesis and stem cell establishment of rabbits, Cloning and Stem Cells, vol. 10, no. 4, p. 523-534, 2008. https://doi.org/10.1089/clo.2008.0023
[6] X. Yue, Y. Zhao, C. Zhang, J. Li, Z. Liu, J. Liu, et al. Leukemia inhibitory factor promotes emt through stat3-dependent mir-21 induction, Oncotarget, vol. 7, no. 4, p. 3777-3790, 2015. https://doi.org/10.18632/oncotarget.6756
[7] S. Bian, Y. Yang, W. Liang, K. Zhang, L. Chen, & Z. Zhang. Leukemia inhibitory factor promotes gastric cancer cell proliferation, migration, and invasion via the lifr–hippo–yap pathway, Annals of the New York Academy of Sciences, vol. 1484, no. 1, p. 74-89, 2020. https://doi.org/10.1111/nyas.14466
[8] Z. Ghanei, N. Mehri, A. Jamshidizad, M. Joupari, & M. Shamsara. Immunization against leukemia inhibitory factor and its receptor suppresses tumor formation of breast cancer initiating cells in balb/c mouse, Scientific Reports, vol. 10, no. 1, 2020. https://doi.org/10.1038/s41598-020-68158-0
[9] Y. Li, H. Zhang, Y. Zhao, C. Wang, Z. Cheng, L. Tang, et al. A mandatory role of nuclear pak4-lifr axis in breast-to-bone metastasis of erα-positive breast cancer cells, Oncogene, vol. 38, no. 6, p. 808-821, 2018. https://doi.org/10.1038/s41388-018-0456-0
[10] H. Zeng, J. Qu, N. Jin, J. Xu, C. Lin, Y. Chen, et al. Feedback activation of leukemia inhibitory factor receptor limits response to histone deacetylase inhibitors in breast cancer, Cancer Cell, vol. 30, no. 3, p. 459-473, 2016. https://doi.org/10.1016/j.ccell.2016.08.001
[11]M. Wang, N. Fer, J. Galeas, E. Collisson, S. Kim, J. Sharib, et al. Blockade of leukemia inhibitory factor as a therapeutic approach to kras driven pancreatic cancer, Nature Communications, vol. 10, no. 1, 2019. https://doi.org/10.1038/s41467-019-11044-9