Bladder cancer is a prevalent malignant tumor, ranking fourth among cancers in men and commonly occurring in women, with men having a 3-4 times higher risk than women [1]. As of 2020, the American Cancer Society estimated approximately 81,400 new cases of bladder cancer in the United States, with around 62,100 cases in men and 19,300 cases in women. There were also 17,980 deaths attributed to bladder cancer, including about 13,050 in men and 4,930 in women. While there has been a slight decline in new cases and associated deaths in women, the incidence rates have decreased in men, although death rates remain relatively stable.
So, what exactly is bladder cancer, and how does it develop? This article will provide a detailed exploration of the molecular biology, formation mechanisms, signaling pathways, and biomarkers associated with bladder cancer.
1. Basic Concepts of Bladder Cancer
1.1 Structure and Function of the Bladder
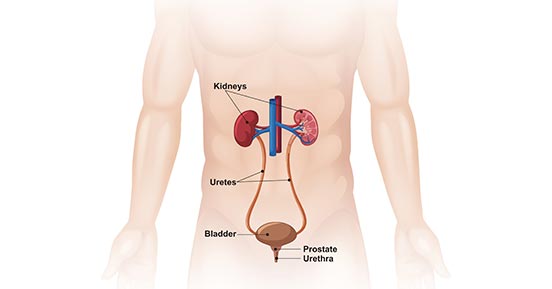
The bladder, a crucial organ in the human urinary system located in the pelvic cavity, plays a pivotal role in storing and expelling urine. Its primary components include the bladder wall, mucosa, and detrusor muscle.
Figure 1. Male urinary system
- Bladder Wall: The bladder wall is composed of three parts—mucosa, muscle layer, and serosa. The mucosa layer, characterized by folds, can expand and contract to accommodate changes in urine volume. The muscle layer contains smooth muscle tissue responsible for propelling urine out. The serosa layer covers the surface of the bladder, providing protection and lubrication.
- Mucosa: The mucosa is the mucous membrane layer located within the bladder cavity. Its surface features folds and small ridges, playing a crucial role in maintaining the stability of urine.
- Detrusor Muscle: Situated at the base of the bladder, the detrusor muscle is a circular smooth muscle responsible for controlling the flow of urine. During urination, the detrusor muscle contracts, opening the urethral opening and facilitating the expulsion of urine.
1.2 Classification and Epidemiological Characteristics of Bladder Cancer
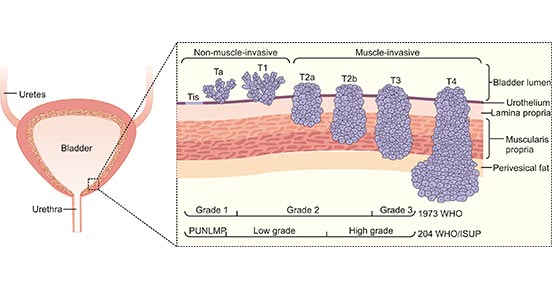
Bladder cancer is classified into two main categories: non-muscle-invasive and muscle-invasive. Non-muscle-invasive bladder cancer typically forms slowly on the surface of the bladder mucosa. Muscle-invasive bladder cancer involves the deeper layers of the bladder wall and exhibits greater invasiveness.
Bladder cancer is more common in males, with the risk being 3-4 times higher than in females, especially in those aged 60 and above. Smoking is a primary risk factor, and other factors include prolonged exposure to chemicals, chronic bladder inflammation, and family history. Bladder cancer has a global incidence, but there are variations among different regions.
1.3 Staging of Bladder Cancer
Understanding the staging of bladder cancer is crucial for determining the severity of the condition, selecting appropriate treatment plans, and predicting patient prognosis. The staging of bladder cancer typically follows the TNM system proposed by the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC).
T (Tumor)
Describes the size and depth of the primary tumor.
| N (Nodes)
Describes the involvement of lymph nodes.
| M (Distant Metastasis)Describes the presence of distant organ metastasis.
|
|
Tx: The size of the primary tumor cannot be assessed.
T0: No evidence of the primary tumor. Ta: Tumor confined to the inner lining of the bladder mucosa, non-invasive.
Tis: Carcinoma in situ, indicating a pre-cancerous lesion.
T1: Tumor invades the submucosal layer (lamina propria) of the bladder.
T2: Tumor invades the muscle layer of the bladder.
T3: Tumor extends into the surrounding fatty tissue.
T4: Tumor extends into adjacent structures, such as the prostate or cervix.
|
Nx: Unable to assess the involvement of lymph nodes.
N0: No evidence of lymph node involvement.
N1: Involvement of a single pelvic lymph node.
N2: Involvement of multiple or bilateral pelvic lymph nodes.
N3: Involvement of lymph nodes along the common iliac artery.
|
Mx: Unable to assess distant metastasis.
M0: No evidence of distant metastasis.
M1: Presence of distant organ metastasis.
|
By combining the TNM system, the specific staging of bladder cancer can be determined, providing guidance to doctors and assisting in the development of the most appropriate treatment plan.
Figure 2. Types and stages of bladder cancer.
*This figure is derived from the publication on Nat Rev Dis Primers. [2]
2. Bladder Cancer Molecular Biology and Formation Mechanism
The formation of bladder cancer is a multi-gene, multi-step process involving various changes in molecular biology. Here are key aspects of the molecular biology and formation mechanism of bladder cancer:
2.1 Role of Tumor Suppressor Genes and Oncogenes
2.1.1 Tumor Suppressor Genes
Tumor suppressor genes normally inhibit cell growth and prevent carcinogenesis. In bladder cancer, common tumor suppressor genes include TP53, RB1, CDKN2A, among others. Mutations or loss of function in these genes may lead to cells losing normal growth regulation, increasing the risk of carcinogenesis.
2.1.2 Oncogenes
Oncogenes normally promote cell growth and differentiation. In bladder cancer, abnormal activation of certain oncogenes may contribute to the excessive proliferation of cancer cells. Genes like Ras, EGFR, MYC, when expressed abnormally or mutated, may be closely associated with the occurrence and development of bladder cancer.
2.2 Aberrant Activation of Signaling Pathways
2.2.1 Ras/Raf/MAPK Signaling Pathway
The Ras/Raf/MAPK signaling pathway regulates cell growth, differentiation, and survival in normal cells. However, in bladder cancer, abnormal activation of this pathway becomes a driving force for the rapid proliferation of cancer cells. Here is a detailed process of this signaling pathway:
Ras Gene Family: The Ras gene family includes HRAS, KRAS, and NRAS, responsible for transmitting external signals to regulate cell growth and differentiation. In bladder cancer, mutations or overexpression of these genes can lead to abnormal activation of Ras proteins.
Raf Kinase: Activated Ras proteins stimulate Raf kinase, triggering the activation of MAP kinase.
MAP Kinase (MAPK): Activated MAP kinase enters the cell nucleus, influencing gene transcription and cell proliferation. In bladder cancer, abnormal activation of MAPK may promote uncontrolled proliferation of cancer cells, leading to tumor formation.
CUSABIO related recombinant protein products:
2.2.2 PI3K/AKT/mTOR Signaling Pathway
The PI3K/AKT/mTOR pathway plays a crucial role in regulating cell survival, proliferation, and metabolism. In bladder cancer, overactivation of this pathway is closely related to abnormal behavior in cancer cells, including drug resistance and malignant growth. Here is a detailed description of this signaling pathway:
PI3 Kinase (PI3K): Activation of PI3K is initiated by external factors such as growth factors. In bladder cancer, overactivation of PI3K leads to the generation of secondary signals within the cell.
Protein Kinase B (Akt): Activated PI3K promotes the phosphorylation of Akt, regulating multiple targets, including cell cycle proteins and apoptosis-related proteins.
mTOR: Activation of Akt ultimately triggers mTOR activation, a critical regulatory point for cell metabolism and proliferation. In bladder cancer, abnormal activation of mTOR may result in abnormal cell metabolism and excessive proliferation.
In summary, the abnormal activation of the Ras/Raf/MAPK and PI3K/AKT/mTOR signaling pathways is a significant driving force in the occurrence and development of bladder cancer. A deeper understanding of these changes helps reveal potential therapeutic targets and aids in the development of more effective treatment strategies.
2.3 Gene Mutations and Epigenetic Changes
The molecular biology of bladder cancer involves various complex changes, including gene mutations and the regulation of epigenetics. Both of these changes play crucial roles in the onset and progression of bladder cancer.
2.3.1 Gene Mutations
In bladder cancer, multiple gene mutations have been identified, some of which are key drivers of cancer development. Here are some common gene mutations:
TP53: Mutations in the TP53 gene are the most common in bladder cancer, and the protein it encodes is a key regulator of cell cycle control and DNA damage repair.
FGFR3: Mutations in the FGFR3 gene are associated with low-grade infiltration in bladder cancer, especially in non-muscle-invasive bladder cancer.
HRAS: Mutations in the HRAS gene, part of the Ras family, are also closely linked to the occurrence of bladder cancer. These gene mutations can lead to abnormal proliferation, survival, and differentiation of normal cells, ultimately promoting the formation of bladder cancer.
2.3.2 Epigenetic Changes
Epigenetics regulates gene expression through processes such as DNA methylation and histone modification. In bladder cancer, abnormal changes in epigenetics affect gene regulation, influencing cell behavior. The main changes include:
DNA Methylation: Changes in DNA methylation levels in bladder cancer cells may lead to the silencing of certain genes, affecting normal cell function.
Histone Modification: Specific histone modifications can alter the accessibility of certain genes, influencing their expression levels and playing a role in the development of bladder cancer.
In conclusion, gene mutations and epigenetic changes interact to drive the onset and progression of bladder cancer. A comprehensive understanding of these molecular-level changes helps identify potential therapeutic targets and provides a theoretical basis for the design of precision treatments.
3. Bladder Cancer Biomarkers
Biomarkers for bladder cancer are specific molecules or substances that can be measured in the body, playing a crucial role in the early diagnosis, disease monitoring, and treatment of bladder cancer. Here are some common bladder cancer biomarkers, along with their roles, applications, and limitations:
| Biomarkers |
Functions |
Applications |
Limitations |
| CA-125 (Cancer Antigen 125) |
CA-125 is a glycoprotein present in bladder cancer tissues. |
Used for screening, diagnosis, and treatment monitoring of bladder cancer. Combined with other markers or imaging techniques, it can enhance diagnostic accuracy. |
Relatively low specificity and sensitivity; may also be elevated in other diseases. |
| AFP (Alpha-Fetoprotein) |
Alpha-Fetoprotein (AFP) is expressed during fetal development. |
Employed in the diagnosis of non-ovarian origin bladder cancer, such as embryonal carcinoma. Monitors treatment effectiveness. |
May increase in some non-hepatic diseases; limited specificity. |
| Inhibin (Follicle-Stimulating Hormone Inhibitor) |
Used primarily for the diagnosis of rare types of bladder cancer, such as Sertoli cell tumors. Monitors treatment effectiveness. |
May also be elevated in some non-ovarian tumors. |
Limited specificity. |
| NMP22 (Nuclear Matrix Protein 22) |
Involved in the proliferation and apoptosis of bladder cells. |
Utilized for monitoring bladder cancer recurrence and treatment effectiveness. |
Non-specific; may also be elevated in other diseases. |
| UroVysion |
Detects chromosomal abnormalities in bladder cancer cells. |
Applied for early diagnosis and monitoring of bladder cancer. |
May produce false-positive results in some non-cancer cases. |
| Bladder tumor antigen (BTA) stat |
Detects bladder cancer-related antigens. |
Used in bladder cancer screening and monitoring. |
Non-specific; may also be elevated in other diseases. |
| CYFRA 21-1 |
Fragments of cytokeratin 19, associated with bladder cancer. |
Employed to assess the clinical status of bladder cancer. |
May increase in other cancers, such as lung cancer. |
| Urokinase-type plasminogen activator (uPA) |
Facilitates fibrinolysis, contributing to tumor invasion. |
Used in conjunction with other markers for the evaluation of bladder cancer. |
Lacks specificity; may also be elevated in other diseases. |
The combination of these biomarkers and more advanced detection technologies is expected to enhance the early diagnosis of bladder cancer in the future. However, further research and validation are still needed. A deeper understanding of the characteristics of these biomarkers will aid in the development of more effective diagnostic and treatment strategies.
4. Latest Research Advances
The latest research developments in bladder cancer include the establishment of cell culture systems, the study of circular RNA, evaluation of immunotherapy, and the discovery of biomarkers. The following are brief summaries:
Establishment of Cell Culture Systems:
Researchers from the Mullenders team successfully established a long-term culture system for normal mouse urothelial epithelium and developed an efficient human bladder cancer cell culture system [3]. Leveraging this feature, they created a viable biobank containing organoid structures related to bladder cancer from 53 patients.
Study of Circular RNA:
Dong and Su, through RNA sequencing analysis, RNA pull-down experiments, and dual-luciferase reporter gene experiments, elucidated the potential role and molecular mechanisms of circACVR2A in regulating bladder cancer proliferation and metastasis [4-5].
Evaluation of Immunotherapy:
In the field of immunotherapy, scholars conducted a comprehensive review of bladder cancer immunotherapy, particularly balancing BCG and anti-PD-1/PD-L1 therapies [6]. They explained why and how bladder cancer could serve as a model for studying cancer immune responses' predictive factors and mechanisms. This provides insights for improving immunotherapy for bladder cancer and other malignancies, along with predictive biomarkers for response.
Discovery of Biomarkers:
Another study investigated the relationship between IGF2BP3 expression and the prognosis of bladder cancer patients. The results indicated a significant upregulation of IGF2BP3 expression in bladder cancer tissues compared to normal bladder tissues, with high expression closely associated with adverse patient outcomes [7].
Application of Deep Learning Algorithms in Bladder Cancer Detection:
Traditional cystoscopy for bladder cancer detection has certain limitations that may affect recurrence identification. Shkolyar and colleagues are dedicated to developing a deep learning algorithm to enhance bladder cancer detection using cystoscopy [8].
Overall Trends:
Alifrangis and colleagues summarized the progress in molecular pathology, the development of targeted therapies, and the resurgence of immunotherapy, leading to the reclassification of bladder cancer subgroups. They discussed how the integrated features of the genome, transcriptome, and proteome guide future treatment strategies [9].
In conclusion, these studies deepen our understanding of the pathogenesis, treatment approaches, and potential predictive factors for bladder cancer, offering new perspectives and directions for future research and treatment of bladder cancer.
References
[1] Andrew T. Lenis, Patrick M. Lec, Karim Chamie. Bladder Cancer A Review [J]. JAMA. 2020, 324 (19).
[2] Oner Sanli, Jakub Dobruch, Margaret A. Knowles, et al. Bladder Cancer [J]. Nat Rev Dis Primers. 2017.
[3] Jasper Mullenders, Evelien de Jongh, Anneta Brousali,et al. Mouse And Human Urothelial Cancer Organoids: A Tool For Bladder Cancer Research [J]. Proc Natl Acad Sci U S A, 2019.
[4] Wei Dong, Junming Bi, Hongwei Liu, et al. Circular RNA ACVR2A Suppresses Bladder Cancer Cells Proliferation And Metastasis Through MiR-626/EYA4 Axis [J]. MOLECULAR CANCER, 2019.
[5] Yinjie Su, Weilian Feng, Juanyi Shi, et al. CircRIP2 Accelerates Bladder Cancer Progression Via MiR-1305/Tgf-β2/smad3 Pathway [J]. MOLECULAR CANCER, 2020.
[6] Dongkui Song, Thomas Powles, Lei Shi, et al. Bladder Cancer, A Unique Model To Understand Cancer Immunity And Develop Immunotherapy Approaches [J]. THE JOURNAL OF PATHOLOGY, 2019.
[7] Wei Huang, Yuanyuan Li, Cheng Zhang, et al. IGF2BP3 Facilitates Cell Proliferation and Tumorigenesis Via Modulation of JAK/STAT Signalling Pathway in Human Bladder Cancer [J]. JOURNAL OF CELLULAR AND MOLECULAR MEDICINE, 2020.
[8] Eugene Shkolyar, Xiao Jia, Timothy C Chang, et al. Augmented Bladder Tumor Detection Using Deep Learning [J]. EUROPEAN UROLOGY, 2019.
[9] Constantine Alifrangis, Ursula McGovern, Alex Freeman, et al. Molecular and Histopathology Directed Therapy for Advanced Bladder Cancer [J]. NATURE REVIEWS. UROLOGY, 2019.
CUSABIO team. Bladder Cancer: Insights into Molecular Biology,Signaling Pathways, and Biomarkers. https://www.cusabio.com/c-21003.html



Comments
Leave a Comment