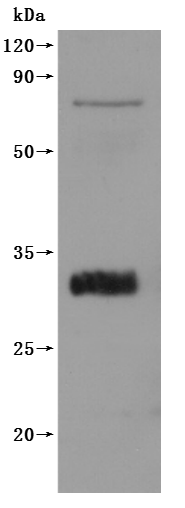
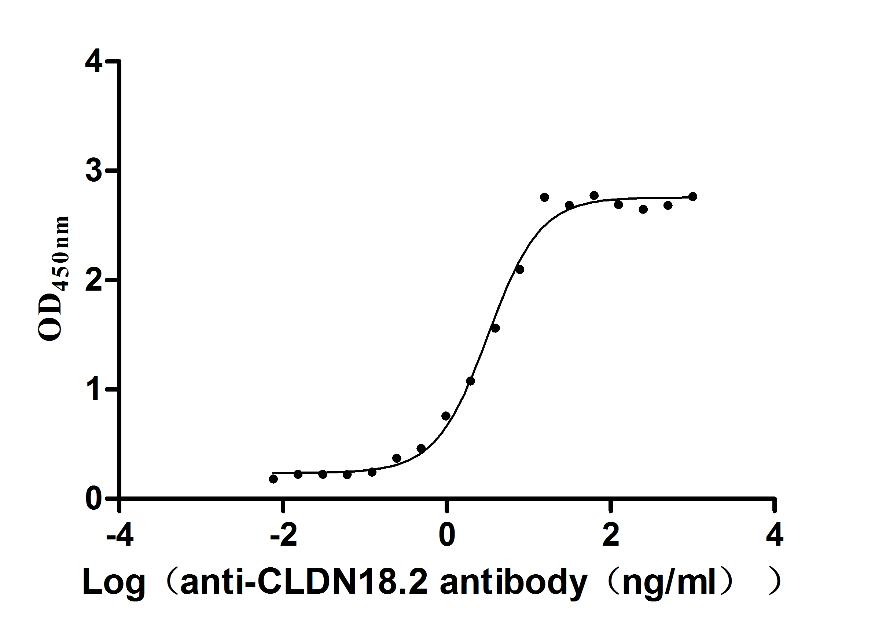
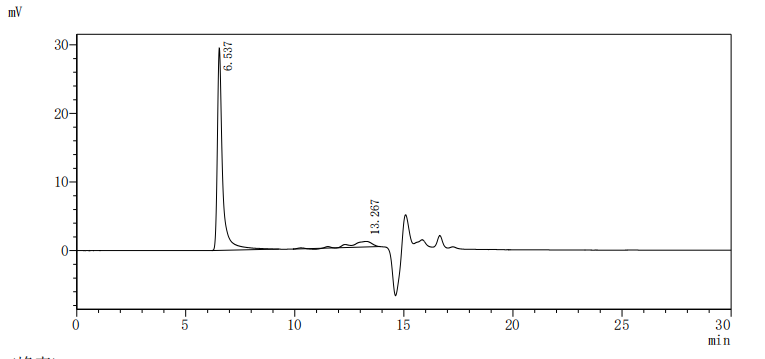
This recombinant Macaca fascicularis CLDN18.2 protein (amino acids 1-261) is produced as a virus-like particle (VLP) in mammalian cells, featuring an N-terminal 6×His tag. The VLP structure mimics native membrane protein conformation while demonstrating high purity (>90% by SEC-HPLC) and low endotoxin levels (<1.0 EU/μg, LAL method). Functional validation via ELISA confirms specific binding to anti-CLDN18.2 antibody (CSB-RA005498A1HU) (EC50: 2.806–3.641 ng/mL at 5 μg/mL immobilization), validating its structural integrity and antigenic properties.
The mammalian expression system ensures proper post-translational modifications critical for CLDN18.2's role in tight junction formation and cell adhesion. The VLP format preserves multi-epitope presentation, making it particularly valuable for evaluating antibody binding kinetics and developing CLDN18.2-targeted biologics. This recombinant protein serves as a robust tool for advancing precision oncology and epithelial biology research.
The CLDN18.2 protein is a member of the claudin family of proteins, which are essential components of tight junctions that regulate the permeability of epithelial and endothelial barriers in various tissues. In Macaca fascicularis (the cynomolgus macaque), the expression and functional roles of CLDN18.2 can be investigated, especially considering that this species serves as a critical model organism in biomedical research due to its close genetic relationship to humans and its relevance in various disease studies [1][2].
In the context of gastrointestinal health and pathology, CLDN18.2 has been highlighted for its specific expression in gastric epithelium. This protein has been primarily studied for its role as a potential biomarker for gastric cancers, which are notably common in humans, and potentially transferable insights could be drawn from the study of this protein in cynomolgus macaques [3][2]. Moreover, the significant similarity in gastrointestinal systems between Macaca fascicularis and humans makes this protein a suitable target for understanding human gastric diseases and the development of therapeutic strategies [3][1].
Additionally, CLDN18.2 has gained attention as a target for antibody-drug conjugates in cancer therapy, particularly in gastric and gastroesophageal junction cancers. Investigating the expression levels and functional implications of CLDN18.2 in Macaca fascicularis can offer insights into tumor biology and the efficacy of novel therapeutics [3][1][2].
The ongoing research on CLDN18.2 in cynomolgus macaques highlights the potential for using this species not only to uncover the basic biological functions of tight junction proteins but also to advance our understanding of their contributions to disease mechanisms and treatment modalities in human medicine.
References:
[1] S. Mariya, F. Dewi, et al. Isolation and characterization of c-c chemokine ligand 7 (ccl7) in cynomolgus macaques. Hayati Journal of Biosciences, vol. 26, no. 3, p. 129, 2019. https://doi.org/10.4308/hjb.26.3.129
[2] S. Laila, D. Astuti, I. Suparto, E. Handharyani, T. Register, & D. Sajuthi. Atherosclerotic lesion of the carotid artery in indonesian cynomolgus monkeys receiving a locally sourced atherogenic diet. Veterinary Sciences, vol. 9, no. 3, p. 105, 2022. https://doi.org/10.3390/vetsci9030105
[3] M. Abdul‐Latiff, F. Ruslin, et al. Continental monophyly and molecular divergence of peninsular malaysia’smacaca fascicularis fascicularis. Biomed Research International, vol. 2014, p. 1-18, 2014. https://doi.org/10.1155/2014/897682