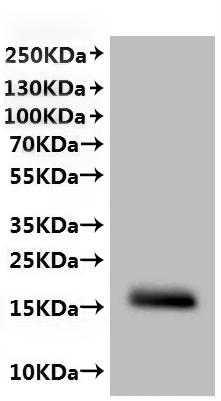
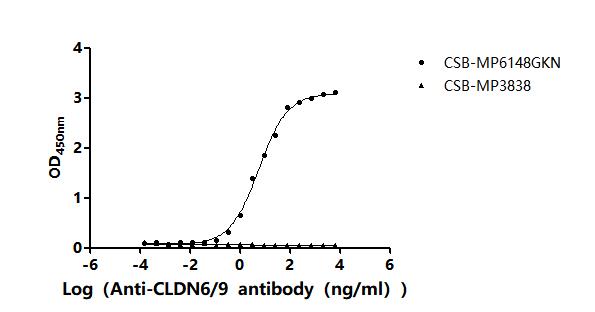
This recombinant Chlorocebus sabaeus CLDN6 protein is produced in mammalian cells as a virus-like particle (VLP), incorporating amino acids 22 to 220 of the native CLDN6 sequence. It carries a C-terminal 10xHis tag for convenient purification and detection. Provided as a lyophilized powder, the protein has an endotoxin level below 1.0 EU/μg, confirmed via the LAL method. Its biological activity is validated through a functional ELISA. It binds to an anti-CLDN6/9 recombinant antibody (CSB-RA005508MA1HU), yielding an EC50 ranging from 4.186 to 6.635 ng/mL. A VLP control (CSB-MP3838) shows no binding, supporting assay specificity.
The CLDN6 protein, a member of the claudin family, is known to contribute significantly to cell adhesion and the formation of tight junctions, which are crucial for maintaining epithelial barrier integrity. In the context of Chlorocebus sabaeus, commonly known as the African Green Monkey, CLDN6 likely regulates paracellular transport and the selective permeability of epithelial layers. Although specific studies focusing solely on CLDN6 in C. sabaeus are limited, relevant data from related research can provide insights into its potential roles and functions.
Firstly, CLDN6 has been implicated in the context of viral infections and interactions with immune responses in primates. For instance, the study by Heigele et al. highlights the role of claudin proteins in viral pathogenesis, particularly how lentiviral proteins can manipulate cellular pathways to downregulate host immune responses, which indirectly implies the importance of CLDN6 [1]. Given that C. sabaeus is an important model organism for simian immunodeficiency virus (SIV) research, understanding how CLDN6 interacts with these viral mechanisms could elucidate its function in viral pathogenesis and immunity.
Furthermore, the genomic analysis of C. sabaeus, particularly in Vero cells derived from this species, has provided a framework for exploring gene expression patterns and the involvement of tight junction proteins like CLDN6 in response to viral infections [2]. Notably, previous studies have underscored the significance of claudins in maintaining barrier function during viral infections, suggesting that manipulating these pathways could enhance our understanding of SIV infectivity and pathogenesis in African Green Monkeys [3].
Additionally, research involving the immune response to SIV in C. sabaeus has indicated the importance of maintaining epithelial integrity, potentially linked to CLDN6 functions. Studies have noted that the claudin protein family contains essential markers in understanding how primate models resist pathogenic infections while often remaining asymptomatic despite SIV presence [4]. Understanding the nuances of CLDN6's expression and its regulatory mechanisms may, therefore, contribute to unraveling the complex interactions between host genetics and viral persistence in this model species.
Lastly, recent analyses of the African Green Monkey microbiome also suggest that microbial interactions can influence claudin gene expression, including CLDN6, further indicating its broader role in health and disease in primate models [5]. Thus, while direct studies on CLDN6 in Chlorocebus sabaeus are scarce, its involvement in tight junction integrity, immunity, and microbiota interactions positions it as a novel target for future research examining host-pathogen dynamics in this species.
References:
[1] A. Heigele, M. Schindler, C. Gnanadurai, J. Leonard, K. Collins, & F. Kirchhoff. Down-modulation of cd8αβ is a fundamental activity of primate lentiviral nef proteins. Journal of Virology, vol. 86, no. 1, p. 36-48, 2012. https://doi.org/10.1128/jvi.00717-11
[2] C. Sakuma, T. Sekizuka, et al. Novel endogenous simian retroviral integrations in vero cells: implications for quality control of a human vaccine cell substrate. Scientific Reports, vol. 8, no. 1, 2018. https://doi.org/10.1038/s41598-017-18934-2
[3] N. Osada, A. Kohara, et al. The genome landscape of the african green monkey kidney-derived vero cell line. Dna Research, vol. 21, no. 6, p. 673-683, 2014. https://doi.org/10.1093/dnares/dsu029
[4] M. Ploquin, Y. Madec, et al. Elevated basal pre-infection cxcl10 in plasma and in the small intestine after infection are associated with more rapid hiv/siv disease onset. Plos Pathogens, vol. 12, no. 8, p. e1005774, 2016. https://doi.org/10.1371/journal.ppat.1005774
[5] B. Jacquelin, G. Petitjean, et al. Innate immune responses and rapid control of inflammation in african green monkeys treated or not with interferon-alpha during primary sivagm infection. Plos Pathogens, vol. 10, no. 7, p. e1004241, 2014. https://doi.org/10.1371/journal.ppat.1004241