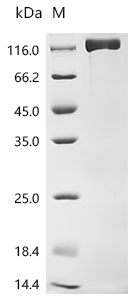
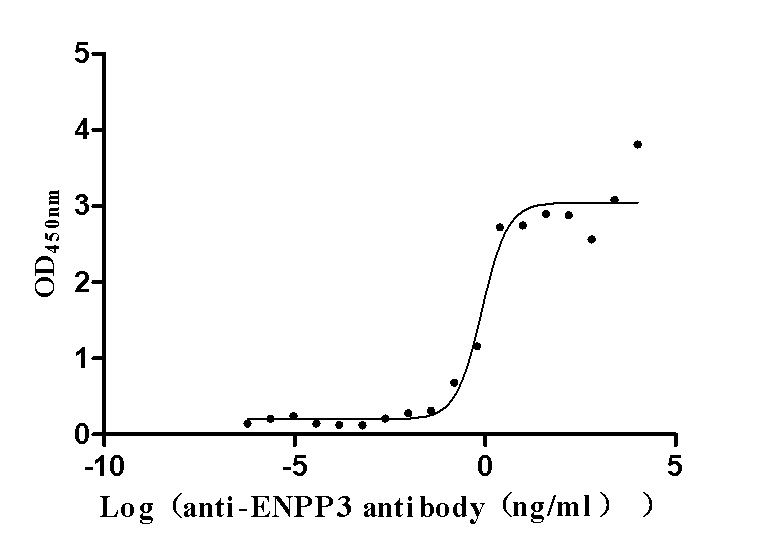
This biologically active recombinant human ENPP3 protein (amino acids 48-875), expressed in mammalian cells with an N-terminal 10×His tag, demonstrates high purity (>95% by SDS-PAGE) and low endotoxin levels (<1.0 EU/μg, LAL method). The protein exhibits strong antigen-binding capability, as validated by ELISA showing specific interaction with anti-ENPP3 antibody (CSB-RA007681MA1HU) at an EC50 range of 0.5355–1.248 ng/mL when immobilized at 2 μg/mL. Mammalian expression ensures proper glycosylation and folding, which are critical for maintaining ENPP3's enzymatic activity in nucleotide metabolism and its role as a therapeutic target in cancers. Presented as lyophilized powder, this preparation ensures stability and easy reconstitution. The N-terminal His tag facilitates purification while preserving functional epitopes. This recombinant ENPP3 protein serves as a key reagent for studies of tumor microenvironments, antibody-drug conjugate development, and investigations into ENPP3-mediated signaling pathways in renal and ovarian cancers.
The human ENPP3 protein belongs to the ecto-nucleotide pyrophosphatase/phosphodiesterase family. It is characterized as a type II transmembrane protein that plays a significant role in purinergic signaling by regulating the metabolism of extracellular nucleotides and their derivatives. Specifically, ENPP3 hydrolyzes various nucleotide substrates, enabling its involvement in crucial biological processes [1][2].
ENPP3 is primarily localized on the cell surface, enhancing its function as an ectoenzyme, participating in the modulation of extracellular signaling events [1][2]. This regulation is particularly important in the context of basal cellular functions and responses to stimuli. In human tissues, ENPP3 is notably expressed in basophils and mast cells, where it assists in cellular activation and differentiation and is considered a reliable marker of basophil activation, which is critical in allergic responses [2][3][4].
Functionally, ENPP3 is linked with the regulation of glycosyltransferase activities, particularly N-acetylglucosaminyltransferase GnT-IX, which is significant for glycan synthesis and cellular interactions involved in endometrial receptivity [1][5]. Its expression is influenced by hormonal fluctuations, particularly progesterone, which underscores its potential role in reproductive biology [6][7].
Beyond its enzymatic activities, ENPP3 has implications in cancer biology, particularly in renal cell carcinoma, where its expression correlates with tumor invasiveness and metastasis. It has been targeted in therapeutic approaches, notably as a specific antigen for antibody-drug conjugates aimed at treating renal tumors, such as AGS16F [8][9]. Furthermore, its expression in reproductive tissues supports its contribution to female fertility, particularly during the menstrual cycle [7][3].
References:
[1] H. Korekane, J. Park, et al. Identification of ectonucleotide pyrophosphatase/phosphodiesterase 3 (enpp3) as a regulator of n-acetylglucosaminyltransferase gnt-ix (gnt-vb). Journal of Biological Chemistry, vol. 288, no. 39, p. 27912-27926, 2013. https://doi.org/10.1074/jbc.m113.474304
[2] R. Borza, F. Salgado-Polo, W. Moolenaar, & A. Perrakis. Structure and function of the ecto-nucleotide pyrophosphatase/phosphodiesterase (enpp) family: tidying up diversity. Journal of Biological Chemistry, vol. 298, no. 2, p. 101526, 2022. https://doi.org/10.1016/j.jbc.2021.101526
[3] B. Hood, B. Liu, et al. Proteomics of the human endometrial glandular epithelium and stroma from the proliferative and secretory phases of the menstrual cycle1. Biology of Reproduction, vol. 92, no. 4, 2015. https://doi.org/10.1095/biolreprod.114.127217
[4] H. Huang and Y. Li. Mechanisms controlling mast cell and basophil lineage decisions. Current Allergy and Asthma Reports, vol. 14, no. 9, 2014. https://doi.org/10.1007/s11882-014-0457-1
[5] Y. Yuan, W. Zhang, et al. Genome-wide selective analysis of boer goat to investigate the dynamic heredity evolution under different stages. Animals, vol. 12, no. 11, p. 1356, 2022. https://doi.org/10.3390/ani12111356
[6] V. Ahmad, S. Yeddula, B. Telugu, T. Spencer, & A. Kelleher. Development of polarity-reversed endometrial epithelial organoids. 2023. https://doi.org/10.1101/2023.08.18.553918
[7] N. Boggavarapu, S. Lalitkumar, et al., Compartmentalized gene expression profiling of receptive endometrium reveals progesterone regulated enpp3 is differentially expressed and secreted in glycosylated form. Scientific Reports, vol. 6, no. 1, 2016. https://doi.org/10.1038/srep33811
[8] W. Zuo and H. Kwok. Development of marine-derived compounds for cancer therapy. Marine Drugs, vol. 19, no. 6, p. 342, 2021. https://doi.org/10.3390/md19060342
[9] F. Doñate, A. Raitano, et al. Ags16f is a novel antibody drug conjugate directed against enpp3 for the treatment of renal cell carcinoma. Clinical Cancer Research, vol. 22, no. 8, p. 1989-1999, 2016. https://doi.org/10.1158/1078-0432.ccr-15-1542