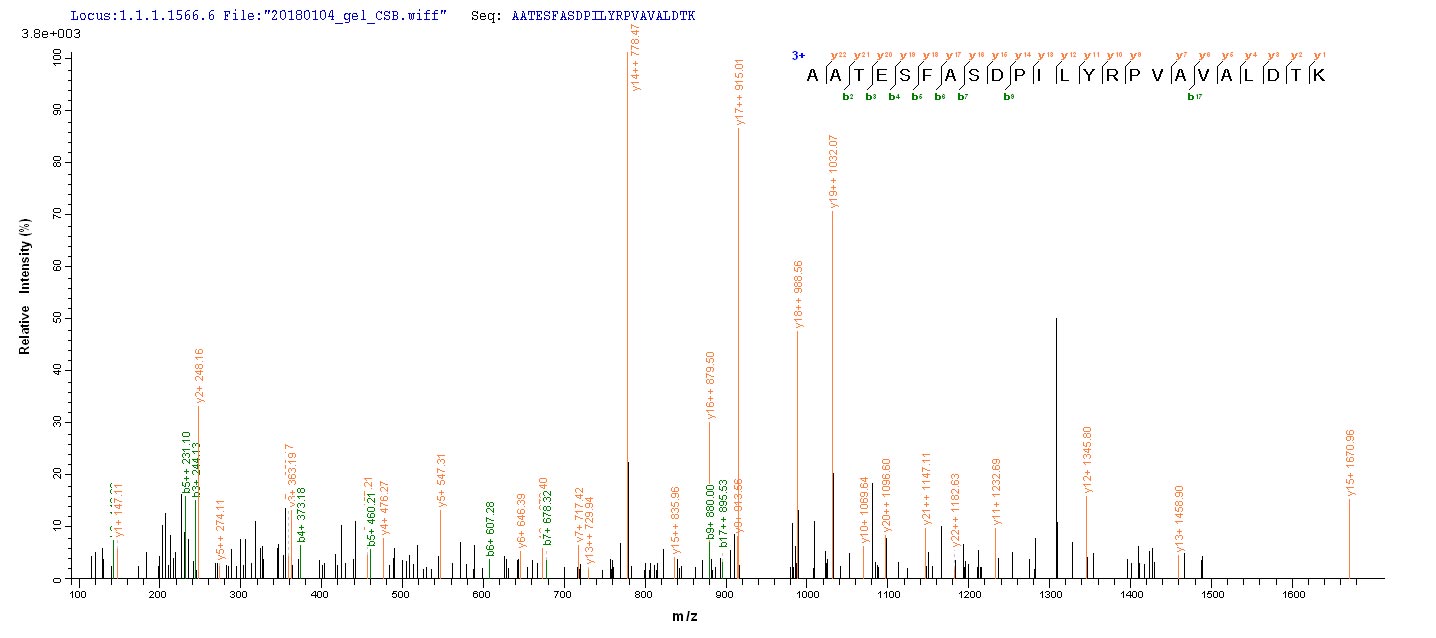
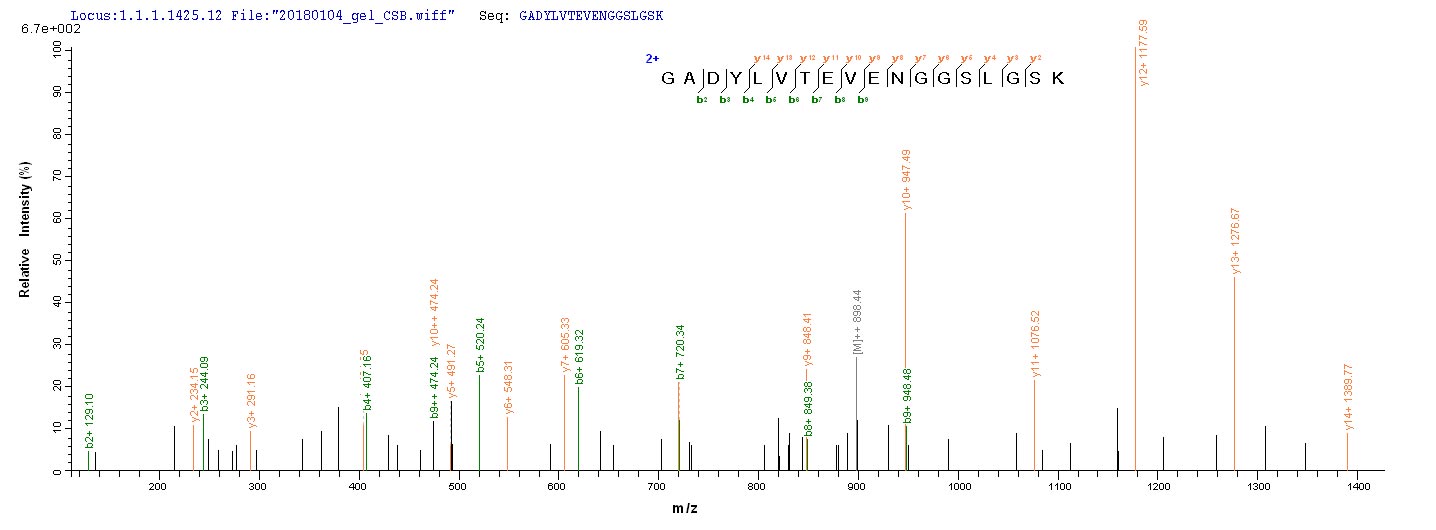
Generating the recombinant rat PKM protein starts with the co-insertion of the gene fragment encoding the 1-531aa of the rat PKM with the N-terminal 10xHis-tag and C-terminal Myc-tag gene into the plasmid, followed by the transfection of the recombinant plasmid into the E.coli, and finally induces the protein expression. The purity of this recombinant rat PKM is over 85% as assessed by SDS-PAGE. It is available in liquid or lyophilized powder form.
Pyruvate kinase M (PKM) is a critical enzyme regulating glycolysis, particularly in the final step where it catalyzes the conversion of phosphoenolpyruvate (PEP) to pyruvate, producing ATP in the process. In mammals, PKM exists in two primary isoforms: PKM1 and PKM2, which arise from alternative splicing of the PKM gene. PKM2 is predominantly expressed in embryonic tissues and cancer cells, while PKM1 is found in tissues requiring high energy, such as the brain and muscle [1][2][3].
The role of PKM2 in cancer metabolism is particularly significant. It is often associated with the Warburg effect, where cancer cells preferentially utilize glycolysis for energy production even in the presence of oxygen, leading to increased lactate production [4][5]. This metabolic reprogramming supporting rapid cell proliferation and tumor growth [6][7]. Studies have shown that the expression of PKM2 can influence various cellular processes, including growth, apoptosis, and metastasis, making it a potential therapeutic target in cancer treatment [8][9][10].
References:
[1] X. Chen, S. Chen, & D. Yu, Protein kinase function of pyruvate kinase m2 and cancer, Cancer Cell International, vol. 20, no. 1, 2020. https://doi.org/10.1186/s12935-020-01612-1
[2] A. Williams, V. Khadka, M. Tang, A. Avelar, K. Schunke, M. Menoret al., Hif1 mediates a switch in pyruvate kinase isoforms after myocardial infarction, Physiological Genomics, vol. 50, no. 7, p. 479-494, 2018. https://doi.org/10.1152/physiolgenomics.00130.2017
[3] Y. Li, S. Teoh, E. Ensink, M. Ogrodzinski, C. Yang, A. Vázquezet al., Cysteine catabolism and the serine biosynthesis pathway support pyruvate production during pyruvate kinase knockdown in pancreatic cancer cells, Cancer & Metabolism, vol. 7, no. 1, 2019. https://doi.org/10.1186/s40170-019-0205-z
[4] X. Li, W. Kim, M. Arif, C. Gao, A. Hober, D. Kotolet al., Discovery of functional alternatively spliced pkm transcripts in human cancers, Cancers, vol. 13, no. 2, p. 348, 2021. https://doi.org/10.3390/cancers13020348
[5] S. Lunt, V. Muralidhar, A. Hosios, W. Israelsen, D. Gui, L. Newhouseet al., Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation, Molecular Cell, vol. 57, no. 1, p. 95-107, 2015. https://doi.org/10.1016/j.molcel.2014.10.027
[6] Z. Gu, J. Xia, H. Xu, I. Frech, G. Tricot, & F. Zhan, Nek2 promotes aerobic glycolysis in multiple myeloma through regulating splicing of pyruvate kinase, Journal of Hematology & Oncology, vol. 10, no. 1, 2017. https://doi.org/10.1186/s13045-017-0392-4
[7] J. Mukherjee, J. Phillips, S. Zheng, J. Wiencke, & S. Ronen, Pyruvate kinase m2 expression, but not pyruvate kinase activity, is up-regulated in a grade-specific manner in human glioma, Plos One, vol. 8, no. 2, p. e57610, 2013. https://doi.org/10.1371/journal.pone.0057610
[8] W. Yang and Z. Lu, Pyruvate kinase m2 at a glance, Journal of Cell Science, 2015. https://doi.org/10.1242/jcs.166629
[9] Y. Li, Z. Zhao, W. Liu, & X. Li, Snhg3 functions as mirna sponge to promote breast cancer cells growth through the metabolic reprogramming, Applied Biochemistry and Biotechnology, vol. 191, no. 3, p. 1084-1099, 2020. https://doi.org/10.1007/s12010-020-03244-7
[10] L. Ni, B. Lin, L. Hu, R. Zhang, F. Fu, M. Shenet al., Pyruvate kinase m2 protects heart from pressure overload‐induced heart failure by phosphorylating rac1, Journal of the American Heart Association, vol. 11, no. 11, 2022. https://doi.org/10.1161/jaha.121.024854